Major Mechanisms of Thrombus Formation
Thrombogenesis in ACS is often initiated by complications of vulnerable atherosclerotic plaques that are prone to rupture, mainly due to their elevated content of lipids and apoptotic cells, leading to a necro-fatty core and a thin-cap fibrous coverage.8 The loss of endoth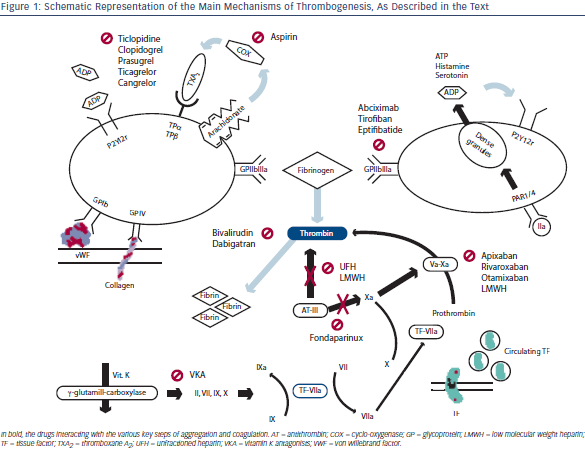 elial coverage leads to exposure of several factors that trigger the two main pathways of thrombus formation: coagulation and platelets activation. Figure 1 summarises the major actors of thrombogenesis as well as the drugs sites of interaction with the physiological process. Animal models suggest that platelets recruitment in the injury site and their activation are driven by two main mechanisms: the first may be independent of the coagulation cascade. In fact, exposure of the von Willebrand factor (vWF) and subendothelial collagen, captures the platelets respectively by interaction with platelet glycoprotein (GP) Ib-V-IX and GP VI, which seems to be also the main responsible for their thrombin-independent activation. The second pathway involves tissue factor (TF) release and its binding to factor VIIa that activates factor IX, leading to thrombin generation and, finally, platelets activation via protease-activatedreceptor 1 (PAR-1) that also triggers amplification of the response by release of adenosine diphosphate (ADP), serotonine and thromboxane A2 (TXA2).1 In particular, TXA2 is synthetised from arachidonate by the cyclo-oxygenase (COX) pathway, while ADP is released by the dense granules of the platelets. Both these important mediators enhance platelets activation by autocrine and paracrine actions, interacting with G-protein coupled receptors, namely TPα and TPβ for TXA2, and P2Y1 and P2Y12 for ADP. All the effectors finally trigger aggregation activating the IIb/IIIa (αIIbβ3) integrin receptor for fibrinogen and vWF, but platelet activation is not uniform in the early phases of thrombus formation.10 Therefore, initial thrombus is a dynamic entity, with continuous platelets activation, adhesion and separation, rendering the clot architecture and shape extremely variable.
elial coverage leads to exposure of several factors that trigger the two main pathways of thrombus formation: coagulation and platelets activation. Figure 1 summarises the major actors of thrombogenesis as well as the drugs sites of interaction with the physiological process. Animal models suggest that platelets recruitment in the injury site and their activation are driven by two main mechanisms: the first may be independent of the coagulation cascade. In fact, exposure of the von Willebrand factor (vWF) and subendothelial collagen, captures the platelets respectively by interaction with platelet glycoprotein (GP) Ib-V-IX and GP VI, which seems to be also the main responsible for their thrombin-independent activation. The second pathway involves tissue factor (TF) release and its binding to factor VIIa that activates factor IX, leading to thrombin generation and, finally, platelets activation via protease-activatedreceptor 1 (PAR-1) that also triggers amplification of the response by release of adenosine diphosphate (ADP), serotonine and thromboxane A2 (TXA2).1 In particular, TXA2 is synthetised from arachidonate by the cyclo-oxygenase (COX) pathway, while ADP is released by the dense granules of the platelets. Both these important mediators enhance platelets activation by autocrine and paracrine actions, interacting with G-protein coupled receptors, namely TPα and TPβ for TXA2, and P2Y1 and P2Y12 for ADP. All the effectors finally trigger aggregation activating the IIb/IIIa (αIIbβ3) integrin receptor for fibrinogen and vWF, but platelet activation is not uniform in the early phases of thrombus formation.10 Therefore, initial thrombus is a dynamic entity, with continuous platelets activation, adhesion and separation, rendering the clot architecture and shape extremely variable.
Coagulation is the other major pathway of thrombus formation, and its activation relays mainly on the release and exposure of TF, a membrane protein that is constitutively expressed by fibroblasts and smooth muscle cells, while it is inducible on monocytes and endothelial cells.11 Furthermore, it is known that TF is also present in circulating blood, on the surface of tiny cell-derived vescicular structures that can be captured by the P-selectin expressed by activated platelets.12 This form of TF is usually inactive, and can be cleaved into the active form by disulphide isomerase expressed by platelets and endothelial cells at the injury site, probably being the main enzyme responsible for fibrin deposition inside the thrombus.13 Once in the active form, TF forms complexes with circulating factor VIIa that are able to activate factors VII, IX and X: the binding of factor Xa to factor V promotes the production of small quantities of thrombin that probably ignites the burst of the subsequent coagulation process.14 In fact, the initial small amount of thrombin can activate both factors V and VII, leading to efficient conversion of prothrombin into thrombin itself by the more active complex Xa-Va. As thrombin availability is the main limiting factor for the coagulative chain burst, the final step of the conversion of fibrinogen into fibrin is greatly enhanced after the early ignition phase, and this process further propagates the thrombus, eventually leading to pathological thrombosis.