Kidney
ADHF is associated with a high incidence (~25 %) of AKI, often superimposed on pre-existing chronic renal impairment. In unselected series half of patients with CHF have an estimated eGFR below 60 ml/min/1.73m2.25,26 AKI usually manifests in the first few days of admission and is most commonly regarded as type 1 cardiorenal syndrome defined as acute impairment of renal function secondary to acute cardiac insufficiency. Both background renal dysfunction and any acute decrement portend a worse prognosis in ADHF.25–28 Since 2000, reports from several large series consistently indicate that AKI, defined as an increment in plasma creatinine of over 0.3 mg/dl (i.e. 26.4 μmol/l), is associated with a near doubling of mortality at 1 year and increments in creatinine as little as 0.1 mg/ dl (9 μmol/l) are associated with worse prognosis.28
Although efforts to date to target AKI in ADHF with specific pharmacotherapies have been disappointing,29 awareness of developing kidney injury in ADHF can trigger 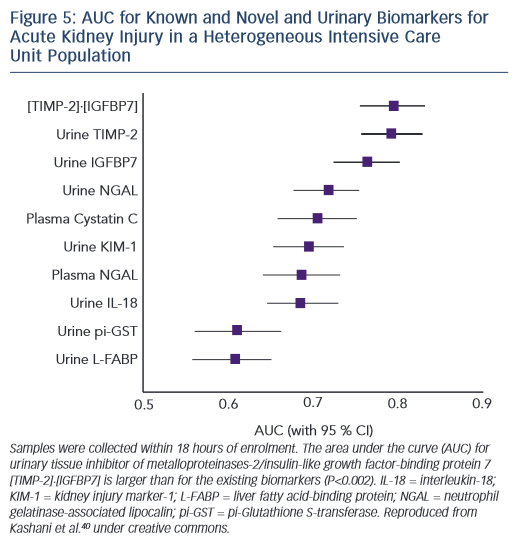 supportive measures (avoidance of nephrotoxic drugs and contrast media, avoidance of hypotension, careful titration of any measures influencing volume and pressure homeostasis) and resulting in improved outcomes.30 Unfortunately, dependence on serial measurement of plasma creatinine to monitor renal status delays awareness of AKI by 24–72 hours. A major cause of failed intervention trials in AKI is delayed intervention resulting from the delay inherent in the use of creatinine as the diagnostic surrogate of reduced GFR, and the misconception that loss of filtration function is an early rather than a late sign of renal injury.31 Further, many patients with a near-normal serum creatinine level on admission with ADHF have evolving AKI, not apparent due to absence of information on pre-admission baseline values. In addition, whether AKI in HF from hypoperfusion differs from injury resulting from renal venous congestion is unclear.32 Hence there is an important unmet need for early markers of AKI in AHF in order to allow timely introduction of supportive measures and to target later experimental therapies for AKI in ADHF.
supportive measures (avoidance of nephrotoxic drugs and contrast media, avoidance of hypotension, careful titration of any measures influencing volume and pressure homeostasis) and resulting in improved outcomes.30 Unfortunately, dependence on serial measurement of plasma creatinine to monitor renal status delays awareness of AKI by 24–72 hours. A major cause of failed intervention trials in AKI is delayed intervention resulting from the delay inherent in the use of creatinine as the diagnostic surrogate of reduced GFR, and the misconception that loss of filtration function is an early rather than a late sign of renal injury.31 Further, many patients with a near-normal serum creatinine level on admission with ADHF have evolving AKI, not apparent due to absence of information on pre-admission baseline values. In addition, whether AKI in HF from hypoperfusion differs from injury resulting from renal venous congestion is unclear.32 Hence there is an important unmet need for early markers of AKI in AHF in order to allow timely introduction of supportive measures and to target later experimental therapies for AKI in ADHF.
Many recently identified biomarkers of renal cellular damage, such as kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL) and interleukin-18 (IL-18), facilitate earlier detection of AKI following ischaemic and nephrotoxic kidney injury.31,33–35 None have entered routine clinical practice. It is unlikely a single biomarker could identify AKI due to aetiological diversity and clinical uncertainty regarding time of onset of renal insult.11
NGAL has claimed particular attention in recent years, but published reports consistently indicate the AUC for detection of AKI in ADHF using urine or serum NGAL is ~0.7.36–39 In order to offer meaningful clinical advantage, markers generally require AUCs in the order of 0.75 or more. In this regard exciting recent data from a trial in patients with heterogenous forms of serious acute disease indicate that the cell cycle markers insulin growth factor binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases type 2 (TIMP-2) perform at this level with AUC for detection of AKI of ~0.8 (see Figure 5).40 These new markers have not been tested specifically in an ADHF population.