Introduction on Stroke Prevention in Atrial Fibrillation – The Use of NOACs in Everyday Clinical Practice
Non-antivitamin K oral anticoagulants (NOACs) are no longer ‘new’ drugs: they already are established. Since randomised controlled trials (RCTs) usually enrol highly selected patient populations, the best way to demonstrate new drug efficiency/cost-effectiveness is to use long-term and ‘real-world’ data. In order to understand the NOAC experience in real life properly, one must first understand how the substrate for such therapy was modified, i.e. what is the awareness and attitude towards stroke prevention in atrial fibfrillation (AF) in everyday clinical practice. Several important registries, 1–4 with different collection designs, have demonstrated that despite the considerable increase in the awareness of AF-related risk of stroke in the last years, the percentage of undertreated patients remains high. This also includes the AF patients with lower risk (CHA 2 DS 2 VASc score of one in men and two in women) who also require anticoagulant therapy. 5 There is also a percentage of over-treated patients (CHA 2 DS 2 VASc of 0) and an excess of aspirin use despite of the demonstrated lack of preventive effect of this drug in AF 6 and increased bleeding risk when 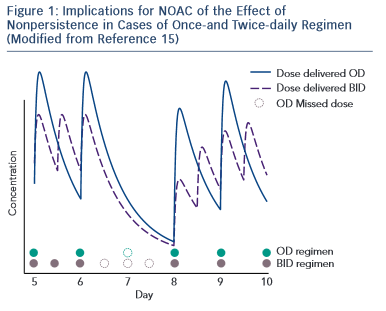 associated to oral antivitamin K anticoagulants (AVKs) or NOACs. A third conclusion is that despite an increase of worldwide approval of NOAC they are still underused. RELY-ABLE study was an open-label prolongation of the RE-LY study with a 28-month extension of the parent study follow-up beyond 4 years of ongoing treatment. The results were consistent with those of RE-LY with an impressive low rate of intracerebral bleeding that was sustained throughout the study. 7 XANTUS was a prospective, single-arm, observational, non-interventional phase IV study intended to collect real-world data on adverse events in patients with non-valvular AF treated with rivaroxaban and to determine the safety profile of rivaroxaban across the broad range of patient risk profiles encountered in routine clinical practice. 8 Again the stroke and bleeding rates were low in patients treated with NOAC. An interesting post-hoc pooled analysis of the RE-LY study according to the European label recommendations failed to demonstrate a difference compared with the original RE-LY study. 9 An extensive real-life analysis included 134,414 elderly patients treated with 2 dosages of dabigatran (150 mg and 75 mg – not approved in Europe) and warfarin and collected 2,715 events. The results confirmed global risk benefit observed in RE-LY (20 % reduction in stroke and 66 % reduction in devastating intracerebral haemorrhage compared with warfarin) but also confirmed the slight increase in gastrointestinal haemorrhage. 10 A somewhat similar pharmacovigilance study conducted by the US Department of Defense electronic healthcare records that included 27,467 real-life patients treated with rivaroxaban confirmed the low rate of bleeding observed in ROCKET-AF. 11 Adherence to therapy is a very important target in considering NOACs if we need to translate effective therapies in effective disease management in patients and diseases where persistence to warfarin therapy is suboptimal. A once-daily regimen could be an advantage in this regard as demonstrated by several studies, 12,13 which highlight the superiority of rivaroxaban compared with warfarin, dabigatran and apixaban. However, in the case of NOAC the rhythmicity of drug administration could have apparent paradoxical implications. The explanation of this paradox is the relatively short half-life of NOAC. When the drug is administered twice daily (bid), the drug concentration inside the therapeutical interval is more constant with lower trough to peak rate than in the once-daily (od) regimen. This implies that missing one dose (which could happen quite often in real life) of an od regimen will lead to a similar lowering in plasma drug exposure as missing three consecutive doses in a bid regimen, which is less likely in real life 14 (see Figure 1 )
associated to oral antivitamin K anticoagulants (AVKs) or NOACs. A third conclusion is that despite an increase of worldwide approval of NOAC they are still underused. RELY-ABLE study was an open-label prolongation of the RE-LY study with a 28-month extension of the parent study follow-up beyond 4 years of ongoing treatment. The results were consistent with those of RE-LY with an impressive low rate of intracerebral bleeding that was sustained throughout the study. 7 XANTUS was a prospective, single-arm, observational, non-interventional phase IV study intended to collect real-world data on adverse events in patients with non-valvular AF treated with rivaroxaban and to determine the safety profile of rivaroxaban across the broad range of patient risk profiles encountered in routine clinical practice. 8 Again the stroke and bleeding rates were low in patients treated with NOAC. An interesting post-hoc pooled analysis of the RE-LY study according to the European label recommendations failed to demonstrate a difference compared with the original RE-LY study. 9 An extensive real-life analysis included 134,414 elderly patients treated with 2 dosages of dabigatran (150 mg and 75 mg – not approved in Europe) and warfarin and collected 2,715 events. The results confirmed global risk benefit observed in RE-LY (20 % reduction in stroke and 66 % reduction in devastating intracerebral haemorrhage compared with warfarin) but also confirmed the slight increase in gastrointestinal haemorrhage. 10 A somewhat similar pharmacovigilance study conducted by the US Department of Defense electronic healthcare records that included 27,467 real-life patients treated with rivaroxaban confirmed the low rate of bleeding observed in ROCKET-AF. 11 Adherence to therapy is a very important target in considering NOACs if we need to translate effective therapies in effective disease management in patients and diseases where persistence to warfarin therapy is suboptimal. A once-daily regimen could be an advantage in this regard as demonstrated by several studies, 12,13 which highlight the superiority of rivaroxaban compared with warfarin, dabigatran and apixaban. However, in the case of NOAC the rhythmicity of drug administration could have apparent paradoxical implications. The explanation of this paradox is the relatively short half-life of NOAC. When the drug is administered twice daily (bid), the drug concentration inside the therapeutical interval is more constant with lower trough to peak rate than in the once-daily (od) regimen. This implies that missing one dose (which could happen quite often in real life) of an od regimen will lead to a similar lowering in plasma drug exposure as missing three consecutive doses in a bid regimen, which is less likely in real life 14 (see Figure 1 )
This apparent paradox caused by a greater continuity of drug action in a bid regimen could be the basis of a greater need of persistence required for the od than for bid 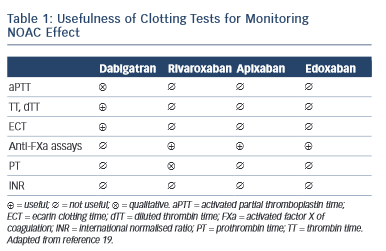 regimen. 15
regimen. 15
A challenge for future research is that currently there is no approved test to guide therapeutical decisions and the routine coagulation monitoring is not mandatory for NOACs. However, in special circumstances (emergent surgery, thrombolysis indication for stroke, haemorrhagic complications, etc.) the clinician needs an informative test. Currently available tests have variable impact depending on NOAC. 16–18 For dabigatran, the prothrombin time (PT) is too insensitive, the thrombin time (TT) is too sensitive and the activated partial thromboplastin time (aPTT) is prolonged under dabigatran, but the relation is curvilinear and reagent-dependent. The diluted thrombin time (Hemoclot) and the ecarin clotting time (ECT) are more suitable but unfortunately with reduced availability. For practical purposes, the most commonly used is the aPTT: a prolonged aPTT indicates that the patient is within therapeutical dabigatran concentration. For rivaroxaban and apixaban the situation is more difficult. The direct anti-Xa activity assay is consistent with plasma concentration, but the test is not widely available. The PT has some sensitivity for rivaroxaban but not for apixaban (see Table 1 )