Direct Catecholamine-mediated Myocardial Stunning
A consistent finding across several mammalian species, including humans, is a higher sympathetic nerve density in the basal myocardium compared with the apex. Conversely, there is an apical-basal gradient of adrenergic receptors ( AR). These opposing sympathetic nerve andAR gradients allow balanced myocardial responses to sympathetic activation under low and medium levels of activation, but at the highest levels epinephrine release from the adrenal glands would perfuse the heart via the circulation and the receptor gradient would determine the response. The second element of the hypothesis proposed is that epinephrine at low and medium doses is a positive inotrope, but at the highest doses it becomes a negative inotrope via the 2AR, by activating a switch from the Guanine Nucleotide binding protein stimulation to the cardioinhibitory Guanine Nucleotide binding protein inhibition (Gi) secondary messenger.38,39 This could explain the transient negative inotropism observed in the apical myocardium after exposure to very high levels of circulating epinephrine, such as those reported in Takotsubo syndrome patients following triggers of extreme stress. Activation of the 2AR-Gi pathway is cardioprotective and may minimise the toxic effects of excessive catecholaminergic stimulation upon the myocardium. This hypothesis was tested in a rodent model in which high-dose epinephrine induced reversible apical hypokinesia and it was found this Takotsubo model could be prevented by prior treatment with pertussis toxin, which deactivates Gi signalling.40. Combinations of these hypotheses form the most likely causes of the cardiac response to severe stress.41
Clinical Presentation and Diagnosis
Individuals with Takotsubo syndrome typically present with acute and unstable chest pain of cardiac origin (angina), breathlessness, palpitations due to sinus tachycardia or arrhythmia and in the more severe cases presyncope or syncope secondary to ventricular tachyarrhythmias, severe LVOTO and/or cardiogenic shock. The majority of diagnoses of Takotsubo syndrome are made at cardiac catheterisation when diagnostic coronary angiography demonstrates the absence of culprit coronary artery disease to explain the presenting symptoms, ECG changes and regional wall motion abnormalities.
Once the diagnosis of Takotsubo syndrome is confirmed, early cardiac imaging and cardiac biomarkers are helpful to exclude myocardial infarction and to further stratify risk. This strategy is to be recommended to allow patients with a higher-risk phenotype to be kept under observation with appropriate monitoring in a coronary care or high- dependency care setting, while those with lower risk can be cared for in a non-specialist ward and discharged once baseline imaging and risk stratification has been completed and the patient is asymptomatic, free of arrhythmias and haemodynamically stable.
When the clinical picture is uncertain, cardiac magnetic resonance imaging with late gadolinium enhancement often clarifies the presence or absence of acute myocardial infarction in a typical coronary distribution.18,19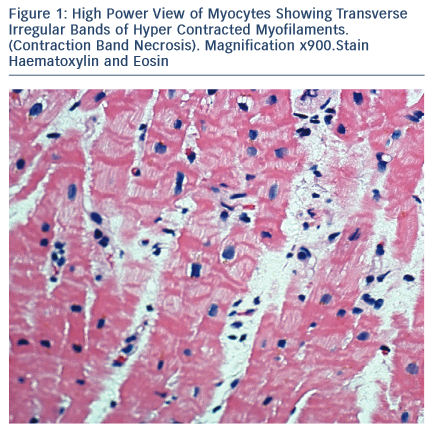
Pathology
The majority of Takotsubo syndrome cases survive. The rare cases that have undergone biopsy during the acute phase show contraction- band necrosis in myocytes as evidence of catecholamine-triggered cardiomyocyte calcium overload (see Figure 1).42 This can be can be fatal due to the complications of cardiogenic shock, arrhythmia or cardiac rupture and can be readily detected post-mortem in a confirmed case of Takotsubo syndrome. More difficult is the case of sudden cardiac death in the community with a stressful precipitant and a structurally normal heart and coronary arteries.43–45
In addition to inherited arrhythmic sudden death syndromes known to be triggered by stress (e.g. catecholaminergic polymorphic ventricular tachycardia), acute Takotsubo syndrome with ventricular fibrillation or asystole should be considered if the heart is normal. There may be dilatation or oedema in the distribution characteristic of an anatomical variant of Takotsubo syndrome, but if post-mortem examination is delayed then non-specific contraction may prevent this assessment.46–48 A hypercontracted left ventricle at autopsy, with increased wall thickening, but a normal heart weight has been reported in the context of severe stress (death in systole). Contraction-band necrosis of individual myocytes with interstitial oedema and a mixed inflammatory cell infiltrate are described in this phenomenon.42,49,50 Cases of idiopathic infarction with normal coronary arteries resulting in sudden death may also be due to Takotsubo.51
Complications and Acute Prognosis
A variety of complications can occur in ≤52 % of Takotsubo patients.8,52,53
Right Ventricular Involvement
Patients with biventricular involvement generally take a more severe clinical course. RV involvement assessed by ECG or magnetic resonance imaging has been reported in 18–34 % of the patients and is associated with older age, a lower LV ejection fraction, a higher frequency of heart failure, pleural effusion and a longer hospital stay.8,54
Acute Heart Failure
Systolic heart failure is the most common complication in the acute phase of Takotsubo syndrome, occurring in 12–45 % of patients.8,16,28,53,55 Independent predictors for the development of acute heart failure are advanced age, low LV ejection fraction on presentation, higher admission and peak troponin levels and a physical stressor preceding the onset of Takotsubo syndrome. In some patients pulmonary oedema due to acute LV dysfunction is aggravated by mitral regurgitation and/or LV outflow tract obstruction.