Diuretic Resistance
Diuretic resistance is a common problem in HF patients. Removal of excessive fluid is usually achieved by a combination of salt restriction and loop diuretics, but in some cases congestion persists despite adequate diuretic therapy. This has been termed diuretic resistance. The prevalence of diuretic resistance in the HF population is unknown due to the heterogeneity of the populations studied, the frequent comorbidity, the different treatment regimens, as well as to the different definitions used in various clinical trials. In a retrospective analysis of 1,153 patients with advanced HF, 402 patients had diuretic resistance (defined in this study as requirement of furosemide >80 mg or bumetanide >2 mg daily).20 Diuretic resistance was independently associated with total mortality, sudden death and pump failure death. Loop diuretics are ‘threshold drugs’. HF shifts the dose-response curve for loop diuretics downward and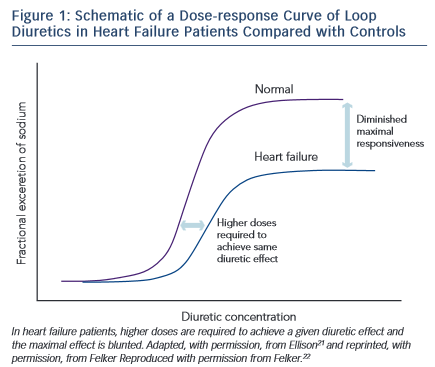 to the right. Thus a higher starting dose of loop diuretics is needed in order to achieve the same level of sodium excretion.21
to the right. Thus a higher starting dose of loop diuretics is needed in order to achieve the same level of sodium excretion.21
The shift of the dose–response curve in HF implicates insufficient dosing as a common cause of a lack of diuretic response (see Figure 1).21,22 The magnitude of natriuresis following a defined dose of diuretics declines over time, even in normal subjects. This is the so-called ‘braking phenomenon’ and it is the result of both haemodynamic changes at the glomerulus as well as adaptive changes in the distal nephron. In a seminal study on rats by Kaissling, furosemide treatment was associated with cell hypertrophy at the distal convoluted tubule, the connecting tubule and the cortical collecting duct.23 These structural changes after furosemide treatment suggest an increase in active transcellular transport capacity of this segment.24 A partial explanation of these anatomical modifications may be the increased stimulation mediated by the renin-angiotensin and sympathetic nervous systems.23 An abrupt increase in diuretic resistance in HF patients may be due to concomitant NSAID use or to an excessive intake of sodium. This may result in renal function deteriorating and development of cardiorenal syndrome.25
A response reduction to diuretic therapy is a common problem in patients with HF and while many studies have tried to give an exact clinical definition of diuretic resistance, others have tried to find a solution to the clinical problems that this causes. Probably the single most used and reproducible marker of cardiovascular congestion is body weight. As a result, HF guidelines advocate daily body weight monitoring in order to detect the pre-symptomatic phase in patients at risk to develop acute decompensated HF.10 An interesting attempt to create a quantitative index of response to diuretic therapy was undertaken by Valente el al.26 This index was obtained by comparing the administered dose of diuretic with the reduction of body weight and was intended to measure its effectiveness. It showed a significant correlation with relevant clinical variables and also highlighted a correlation with adverse events.
In another study, Testani et al. tested a metrical index of diuretic efficiency, which was defined as the net fluid lost per milligram of loop diuretic, thus demonstrating that low diuretic efficiency during decongestive therapy portends poorer long-term outcomes in patients hospitalised with decompensated HF.27
Once correctable variables and blockage of the neuroendocrine system have been excluded, a possible way of overcoming diuretic resistance is to use infusion therapy to avoid the limitations of oral bioavailability. For patients refractory to escalating doses of intravenous diuretics, options include use of continuous infusion rather than intermittent boluses. This strategy was tested in the DOSE study,28 but no significant difference was noted between the two treatment groups.
Another approach is to a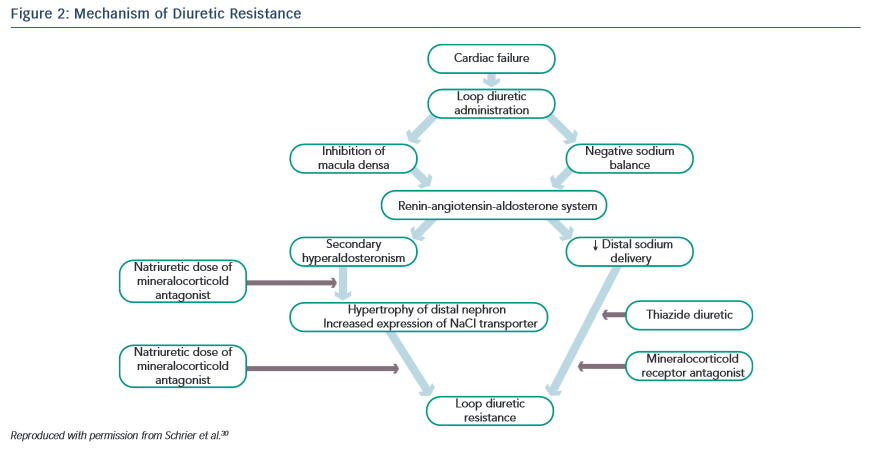 dminister two classes of diuretics together, a loop diuretic combined with a thiazide-like diuretic, thus performing a sequential nephron blockade.29 Various mechanisms explain the success of this combination strategy: the longer half-life of thiazide diuretics helps to counteract the rebound post-diuretic effect (see Figure 2).30 Thiazide-type diuretics inhibit sodium reabsorption in the distal nephron and primarily benefit patients who have distal nephron hypertrophy and hyperfunction due to chronic treatment with loop diuretics. Indeed, inhibiting NaCl transport along the distal tubule counteracts the reabsorption due to hyper-functioning cells in the distal tubule. In addition, they markedly increase the fractional sodium excretion, which is needed to achieve a neutral or negative sodium balance if the GFR is depressed.31
dminister two classes of diuretics together, a loop diuretic combined with a thiazide-like diuretic, thus performing a sequential nephron blockade.29 Various mechanisms explain the success of this combination strategy: the longer half-life of thiazide diuretics helps to counteract the rebound post-diuretic effect (see Figure 2).30 Thiazide-type diuretics inhibit sodium reabsorption in the distal nephron and primarily benefit patients who have distal nephron hypertrophy and hyperfunction due to chronic treatment with loop diuretics. Indeed, inhibiting NaCl transport along the distal tubule counteracts the reabsorption due to hyper-functioning cells in the distal tubule. In addition, they markedly increase the fractional sodium excretion, which is needed to achieve a neutral or negative sodium balance if the GFR is depressed.31
Numerous thiazide-like diuretics have been evaluated in combination with loop diuretics with similar results overall and there is no clear evidence that any single thiazide-like diuretic is superior to another, suggesting a class effect. It has been suggested that metolazone is superior to other thiazide-like diuretics in patients with advanced kidney disease, but other thiazide-like diuretics also increased the response to loop diuretics, even in patients with advanced renal failure. More recently, a small, retrospective, single-centre cohort study compared two of the most commonly used thiazide-like diuretics (oral metolazone and intravenous chlorothiazide) as add-on therapy to loop diuretics and no statistically significant differences in efficacy or safety were found.32 In some European countries, metolazone and chlorothiazide are not available and the most commonly used thiazide-like diuretics for ADHF are hydrochlorothiazide and chlorthalidone. Chorthalidone’s half-life (48–72 hours) is longer than that of hydrochlorothiazide (6–12 hours), which might increase risk of adverse events in patients hospitalised for ADHF. Moreover, head-to- head studies comparing these for treating hypertension described an increased risk of hyponatraemia with chorthalidone.33
For these reasons, hydrochlorothiazide or metalazone could be the diuretic of choice for treating ADHF. The main problem when using sequential nephron blockage is the excessive depletion of water and electrolytes. Chronic thiazide diuretics use is a predictor of worsening renal function in chronic HF and this is of concern, given the adverse prognosis associated with worsening renal function in these patients. Impaired renal function with diuretic therapy can result from direct alterations in glomerular haemodynamics due to neurohormonal and intrarenal feedback mechanisms or from overt volume depletion. To address these common concerns we need to await results of ongoing clinical trials (between these, the ‘Safety and efficacy of the combination of loop with thiazide type diuretics in patients with decompensated HF’, will compare the strategy of sequential block through add-on hydrochlorothiazide versus therapy with loop diuretics alone). As a result of the above considerations, nowadays it is not easy to apply sequential nephron blockage to outpatient settings.34