Approaches
Minimally invasive aortic valve replacement (MIAVR) requires a coordinated effort by the surgeon, perfusionist, anaesthesiologist, cardiologists and nurses to achieve the best clinical outcomes. Intraoperative transesophageal echocardiography (TEE) is used routinely. A pulmonary artery catheter is employed based on patient risk and the specific operation. For both UHS and RAT, a single lumen endotracheal tube is standard. To achieve optimal exposure during RAT, the right lung can be mechanically retracted posteriorly without the need to resort to single-lung ventilation. However, right lung isolation can be useful during the learning curve and in difficult cases using double lumen endotracheal tube or bronchial blocker. To improve emptying of the heart during CPB, vacuum-assisted or kinetic venous drainage is commonly used. The patient is positioned supine and surgically prepped from the neck to mid-thigh for both procedures. External defibrillator pads are placed similarly to redo surgery. The mandatory use and routine interpretation of the intraoperative TEE for de-airing process is a critical step at the end of cardiopulmonary bypass in any MIAVS procedure. The echocardiogram images are visualised and evaluated at the actual time of the operation – this is performed in the same manner as in the conventional full sternotomy approach. Complete de-aired heart will allow weaning of cardiopulmonary bypass and transition to the end of the procedure reducing the microemboli phenomenon.
Upper Hemisternotomy
This is the most c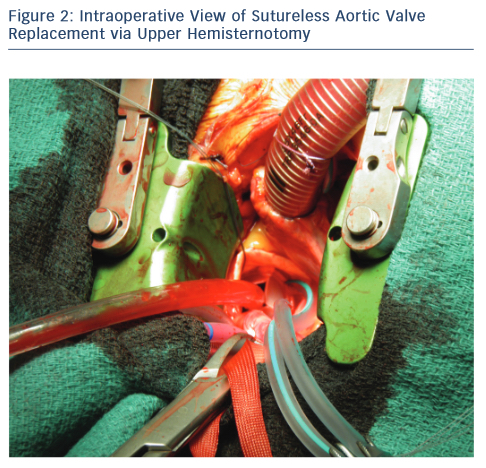 ommon incision used for surgeons for MIAVS. UHS may be the best approach for less-experienced surgeons. This approach implies to split the sternum, the sternotomy incision begins at the sternal notch and is carried down by 5–8 cm to the third or fourth intercostal space on the right. A sternal saw is used and the right internal thoracic artery is spared. A rigid retractor with narrow blades is inserted. Central aortic cannulation is straightforward but should be aimed as distal as possible to provide an unencumbered working space. Venous cannulation can either be peripheral or through the right atrial appendage. Myocardial protection is accomplished to the root or directly to the coronary ostia if antegrade is planned. Retrograde cardioplegia can be either directly or peripherally via internal jugular vein if required.18 The left ventricle can be vented directly through the aortic valve using cardiotomy suction or indirectly with a percutaneously placed pulmonary artery vent placed directly in to the pulmonary artery. A transverse aortotomy is placed slightly higher to facilitate its closure and visualisation at the end of operation (see Figure 2). Retraction sutures are placed on the edges of the aortotomy, and at the peak of each commissure to elevate the aortic valve into the centre of the operative field. The remaining steps of the procedure is similar to conventional valve replacement. We find that placement of the aortic valve sutures is facilitated by instruments with long handles and also using a knotting device such as CoreKnot® reduces valve implant time. As the surface of the heart is not readily accessible, de-airing demands meticulous attention to detail and is monitored using TEE, being as described above a critical step in the operation.
ommon incision used for surgeons for MIAVS. UHS may be the best approach for less-experienced surgeons. This approach implies to split the sternum, the sternotomy incision begins at the sternal notch and is carried down by 5–8 cm to the third or fourth intercostal space on the right. A sternal saw is used and the right internal thoracic artery is spared. A rigid retractor with narrow blades is inserted. Central aortic cannulation is straightforward but should be aimed as distal as possible to provide an unencumbered working space. Venous cannulation can either be peripheral or through the right atrial appendage. Myocardial protection is accomplished to the root or directly to the coronary ostia if antegrade is planned. Retrograde cardioplegia can be either directly or peripherally via internal jugular vein if required.18 The left ventricle can be vented directly through the aortic valve using cardiotomy suction or indirectly with a percutaneously placed pulmonary artery vent placed directly in to the pulmonary artery. A transverse aortotomy is placed slightly higher to facilitate its closure and visualisation at the end of operation (see Figure 2). Retraction sutures are placed on the edges of the aortotomy, and at the peak of each commissure to elevate the aortic valve into the centre of the operative field. The remaining steps of the procedure is similar to conventional valve replacement. We find that placement of the aortic valve sutures is facilitated by instruments with long handles and also using a knotting device such as CoreKnot® reduces valve implant time. As the surface of the heart is not readily accessible, de-airing demands meticulous attention to detail and is monitored using TEE, being as described above a critical step in the operation.
Right Anterior Mini Thoracotomy
RAT avoids sternotomy and is associated with a limited skin incision. However, the operative field is smaller and the aortic valve sits deeper within the wound. Exposure is enhanced by minimising cannula traffic within the incision via peripheral access, coupled with strategic placement of pericardial sutures. This approach is typically performed with a 4–6 cm incision through the second or third intercostal space.
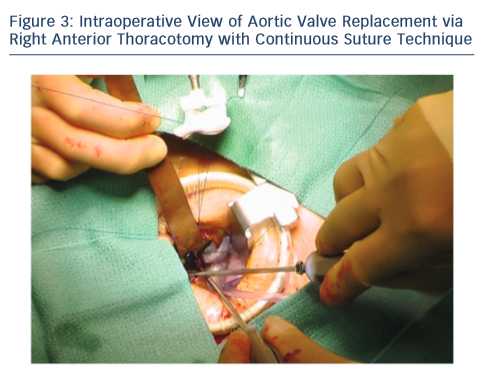
Upon entering the pleural space, the right mammary vessels are usually ligated and divided. The third or fourth rib can be dislocated from the sternum to enhance exposure with the goal of visualising the tip of the right atrial appendage. A soft tissue retractor is inserted into the wound followed by a rigid retractor with narrow blades (see Figure 3). Cannulation for cardiopulmonary bypass can be central but usually peripheral. The crossclamp is applied directly through the incision or from an alternative port; however, it can also be performed peripherally with an endoclamp. Myocardial protection with antegrade cardioplegia is delivered through the root or directly to the coronary ostia. Retrograde cardioplegia could be also delivered peripherally through percutaneous jugular vein catheter into the coronary sinus. Technical details of aortotomy, prosthetic valve implantation and aortotomy closure are identical to UHS. The aortic valve is excised in the usual fashion; however, the right coronary cusp sutures are placed first and retracted to facilitate exposure. At the end of the procedure, a small chest drainage tube (e.g. Blake) is inserted in the right pleural space through a separate intercostal space. Pericardium is left open. The disarticulated rib is reattached to the sternum using non-absorbable, braided suture. To avoid lung herniation, the ribs are then reapproximated using further non-absorbable braided sutures.
Importantly, if exposure with either UHS or RAT is inadequate, then conversion to full sternotomy should be considered. This ensures that valve replacement can be completed safely using an approach familiar to the surgeon.