Transcatheter aortic valve implantation (TAVI) is evolving rapidly with an exponential growth in the number of procedures in worldwide.1,2 As worldwide experience with this modality increases, more and more patients are bein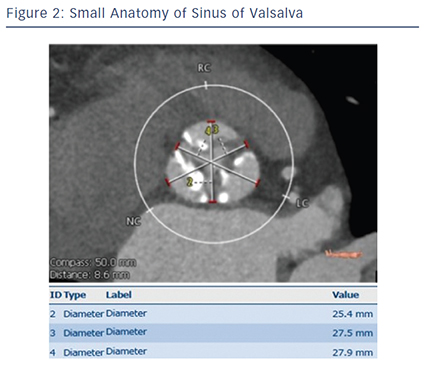 g offered this alternative to open surgery for the treatment of severe, symptomatic aortic stenosis (AS). Although this technique has reached relative maturity, further optimisation of patient selection and device implantation is essential for achieving im
g offered this alternative to open surgery for the treatment of severe, symptomatic aortic stenosis (AS). Although this technique has reached relative maturity, further optimisation of patient selection and device implantation is essential for achieving im proved prognosis.
proved prognosis.
Patients of small body size (e.g. body surface area <1.4 m2) typically have a smaller annulus size and smaller vascular access. Smaller annulus (e.g. annulus area <300 cm2) may increase the risk of annulus rupture due to relative valve oversizing.3 Annulus rupture or perforation is a rare but catastrophic complication of TAVI associated with a high risk of death, and has been reported to be between 0–1.1 %.4,5 Ag gressive device oversizing and large calcifications in the epicardial fat area of the annulus were reported as risk factors for annulus rupture.3,5,6 Our previous report showed a trend towards higher incidence (2.3 %) of annulus rupture in patients with small body size.7 Accurate measurement of the aortic root using multidetector CT is crucial for appropriate device sizing.8–10 A smaller valve such as a 20 mm balloon-expandable transcatheter heart valve should also contribute to the reduction of annulus rupture in patients with smaller annulus (see Figures 1–3).11
gressive device oversizing and large calcifications in the epicardial fat area of the annulus were reported as risk factors for annulus rupture.3,5,6 Our previous report showed a trend towards higher incidence (2.3 %) of annulus rupture in patients with small body size.7 Accurate measurement of the aortic root using multidetector CT is crucial for appropriate device sizing.8–10 A smaller valve such as a 20 mm balloon-expandable transcatheter heart valve should also contribute to the reduction of annulus rupture in patients with smaller annulus (see Figures 1–3).11