Prosthesis Type
The incidence of AV conduction disturbances as a result of TAVR and the subsequent requirement for permanent pacing differs between the two most widely used bioprostheses, the balloon-expandable Edwards SAPIEN valve (ESV) (Edwards Lifesciences, Irvine, California) and the self-expanding MCP. PPM implantation has been reported with a rate between 5–14.2 % [2,13,17–19] for the former Edwards valves (Edwards SAPIEN and Edwards SAPIEN XT) and 13.3 % for the new Edwards SAPIEN 3 THV20, whereas the need for PPM has been higher with use of the MCP (up to 24 % in the FRANCE-2 [French Aortic National CoreValve and Edwards] registry and 33 % in the UK CoreValve registry)21,15.
Early data showed a more liberal pacemaker implantation strategy after MCP TAVR and resulted in higher PPM rates, whereas newer data of the Advance II trial, which were presented at EuroPCR 2014 (ADVANCE II: Low incidence of permanent pacemaker for self -expandable valve by Petronio A. S. et al. at Euro PCR 2014, May 22, Paris, France) show that PPM rate was reduced to 13.3 % at 30 days’ follow up when MCP was deployed according to best recommendation practice (implantation depths <6 mm). Recent data of the CoreValve Extreme Risk pivotal trial revealed a PPM implantation rate of 21.6 % at 30 days22.
In addition to the above-mentioned THVs, four so-called next-generation devices have been evaluated in recent trials. The multicentre non-randomised DISCOVER trial (100 patients) recently assessed the outcomes of the non-metallic direct-flow medical THV in surgical high-risk patients. The device is fully repositionable and retrievable until polymer exchange. In DISCOVER, the overall PPM rate at 30 days was 17 %23.
The REPRISE studies assessed the outcomes with the new mechanically expanding LOTUS valve, which can be fully retrieved, redeployed or repositioned. The 30-day results of the REPRISE II trial (120 patients), which was a prospective, single-arm, multicentre study showed a PPM rate of 28.6 % at 30 days24, which might be explained by the fact that all patients with borderline aortic annulus had to undergo TAVR with the larger 27 mm prosthesis, since the 25 mm prosthesis was not available at that time.
Thirty-day outcomes from the multicentre European pivotal trial for transapical transcatheter aortic valve implantation with the self-expanding Medtronic engager valve prosthesis (Medtronic, Inc., Minneapolis, MN, USA), which consists of a self-expanding nitinol frame and a bovine pericardial tissue valve, revealed a 30-day PPM rate of 26 %25.
The transapical JenaValve™ THV system showed a 30-day pacemaker implantation rate of 9.1 %26. Other new transcatheter valves are currently under development, and will be evaluated in the near future.
Persistence of Conduction Abnormalities and time to Pacemaker Implantation: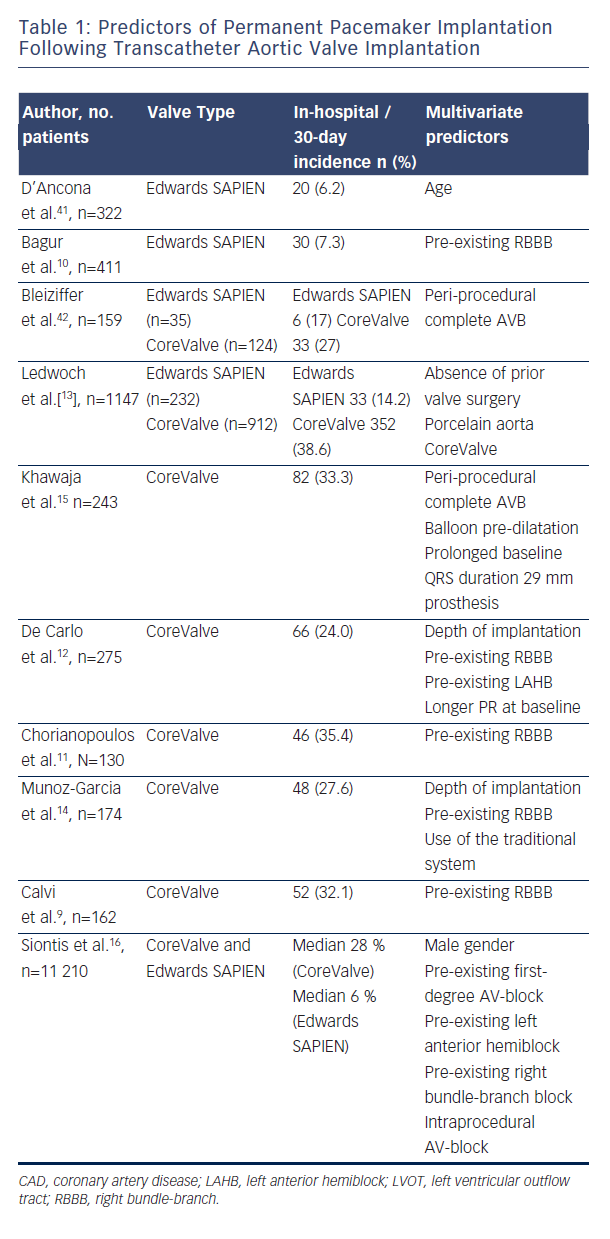
Persistence of conduction disturbances and high-degree AV block over time is not yet clear, and seems to differ between MCP and ESV. Houthuizen reports approximately 40 % of patients developed a new LBBB after TAVI; most of these persisted at follow-up, but MCP had a twofold lower tendency to resolve27. A new LBBB occurs 2.5 times more often after MCP than after ESV implantation and is also associated with reduced recovery28. In another MCP analysis, a proportion of AV conduction disturbances after the intervention has been shown to recover over time at three months of follow-up, and only 40 % of the PPM patients for high-degree AVB still had an AVB underlying their paced rhythm29. Due to the relatively low sample size of these studies, this issue needs further investigation.
Data in relation to the appropriate time point of pacemaker implantation are rare. Simms et al. published a cohort of 100 TAVR patients who received the MCP. Average time to pacemaker implantation was four-and-a-half days (confidence interval: 2.8–6.2)30. In another cohort with the MCP prosthesis (n=270), median time to implantation of a pacemaker was four days (interquartile range [IQR], 2.0 to 7.75 days)15. Bagur et al (n=411) reported that PPM was performed at a median of two days (IQR: 0–4 days) after TAVR with ESV10.
The exact time point for PPM implantation is still an ongoing debate. Another aspect, which has to be kept in mind, is the fact that self-expanding prostheses may lead to delayed injuries of the conduction system. So far, there is no explicit data for the best time point for PPM implantation in patients after Boston Lotus, Medtronic engager or Jena valve implantation.
Pre- and Post-dilatation and Prosthesis Sizing
The close relationship of the conduction system to the aortic annulus may lead to a mechanical interaction between the prosthesis stent frame of the transcatheter valve prosthesis and the left bundle-branch, which in turn may translate into the occurrence of an LBBB and eventually into a higher grade or complete atrio-ventricular block. A study by Lange et al.31 analysed the impact of valvuloplasty balloon catheter size on the need for PPM in a larger cohort of 237 patients without prior pacemaker, who underwent TAVR with the MCP. In this analysis, the overall incidence of PPM was 21.1 %, but was significantly higher when a 25 mm balloon was used (27.1 %) than when a 23 mm or smaller balloon was used (15.4 %) for the balloon valvuloplasty (BAV). When stratified by THV size (26 or 29 mm), there was still a stepwise increase in PPM rate with each increase in balloon size. This association of balloon size with need for a PPM remained significant after multivariable adjustment for baseline patient characteristics. These results suggest that pacemaker rates after TAVR may be reduced by using undersized BAV balloons or even avoidance of pre-dilation. Two randomised studies are currently ongoing to investigate direct TAVR without pre-dilatation with the MCP (SIMPLIFY TAVI Trial; NCT01539746) and the ESV (EASE-IT Trial; NCT02127580). Interestingly, in another study post-dilation after MCP, implantation had no effect on the requirement for PPM. The reason for this observation might be the relatively short time period when the aortic annulus is exposed to high pressure from repeated valvuloplasty31. Post-dilatation after ESV TAVR ranges between 20–41 %32–34 and shows no impact on PPM rate. A recent analysis showed a low post-dilatation rate of 4 % after Edwards SAPIEN 3 TAVR33.
The degree of prosthesis oversizing may lead to a higher incidence of PPM. Schroeter et al. found larger or significantly oversized prostheses to be an independent risk factor for PPM implantation following TAVR with the MCP35. In contrast, a study of Binder et al. with 89 patients receiving a SAPIEN XT THV showed that annular area oversizing was not associated with new conduction disturbances and permanent pacemaker implantation36. If this difference can be explained by the fact that the ESV prosthesis is oversized to a lesser degree to prevent annulus rupture remains unclear and needs further investigation.
Implantation Depths and Approach
Many studies have shown that the CoreValve prosthesis implantation depth is a predictor for PPM. The deeper the CoreValve frame protrudes into the left ventricular outflow tract, the more likely the patient is to develop an LBBB. In an early study by Piazza et al.37, the mean implantation depth was 10.3 mm in those patients with new LBBB versus 5.5 mm in those without LBBB. Another study proposed a cutoff of 6.0 mm as an independent predictor of the development of a high-degree AV block and the requirement for permanent pacing29. This finding was confirmed for the MCP prosthesis by several other recently published studies8,38,39,12. Additionally, implantation of balloon-expandable transfemoral THVs with increased implantation depth is associated with clinically significant new conduction disturbances and permanent pacemaker implantation36. These effects also apply to ESV implantation via a transapical approach40.
The PPM incidence regarding TAVR approach is difficult to assess because patient population and risk profile are often different. A recent meta-analysis of Siontis et al.16 suggested a trend towards lower risk of PPM after MCP TAVR with the transfemoral approach compared with the transsubclavian approach (p=0.07). The same meta-analysis found no difference in PPM risk after ESV TAVR depending on the approach (transapical versus transfemoral; n=2136; risk ratio: 0.89, 95 % CI: 0.64-1.25; p=0.89).