Why Does Stroke Happen After Transcatheter Aortic Valve Replacement?
There are many theories addressing this question, as captured in Table 1. These include procedural risk factors and post-procedural risk factors. Some procedural risk factors are known from experience with balloon valvuloplasty, but some are specific to valves. Serial transcranial Doppler examinations identify high-intensity transient signals (HITS) during transcatheter aortic valve implantation (TAVI), which may serve as a surrogate f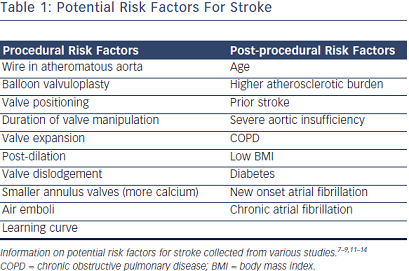 or microembolization.8–10 For example, duration of valve manipulation may be longer and lead to more HITS with self-expanding CoreValve than balloon-expandable Edwards. Of note, atrial fibrillation – both new onset and chronic – are risk factors for stroke in patients with TAVR. These theories and risk factors give rise to additional questions.
or microembolization.8–10 For example, duration of valve manipulation may be longer and lead to more HITS with self-expanding CoreValve than balloon-expandable Edwards. Of note, atrial fibrillation – both new onset and chronic – are risk factors for stroke in patients with TAVR. These theories and risk factors give rise to additional questions.
How Often is Atrial Fibrillation Present in Patients Undergoing Transcatheter Aortic Valve Replacement?
Chronic atrial fibrillation was present at a high rate in both the PARTNER cohort A: high-risk TAVR arm (40.8 %) and cohort B: inoperable TAVR arm (32.9 %). Further analysis of TAVR patients found that atrial fibrillation is a predictor of mortality regardless of type (paroxysmal, persistent or permanent). Atrial fibrillation also predicts mortality regardless of subclass (age, gender, diabetes, renal function, coronary artery disease or left ventricular ejection fraction).15 Further, pre-existing chronic atrial fibrillation was found in one study to predict stroke even after 30 days, with a hazard ratio of 2.84 and a cumulative transient ischaemic attack (TIA)/CVA hazard ratio of 1.91.5 However, this was not the case in other papers, namely by Stortecky et al.,15 likely due to two reasons. Firstly, these studies do not differentiate strokes at >24 hours to later strokes. Secondly, they do not differentiate strokes from 30 days to one-year as a different subgroup. Timing of the cerebrovascular event may be relevant to the cause.
How Often Does New Onset Atrial Fibrillation Occur After Transcatheter Aortic Valve Replacement?
The literature reports a rate between 7.5 and 31.9 %.1,2,5,14,16 Predictive factors for new onset atrial fibrillation include transapical access and large left atrial size.16 The timing of new onset atrial fibrillation is variable. About a third (36.3 %) of new onset atrial fibrillation start during the procedure, but over 50 % of cases start at over a 24 hour time period. Further, the duration of new onset atrial fibrillation is also variable, with >50 % of new onset atrial fibrillation lasting <24 hours. New onset atrial fibrillation in the first 30 days increased the odds of having atrial fibrillation again in the first year.16
Does New Onset Atrial Fibrillation After Transcatheter Aortic Valve Replacement Lead to Stroke?
There is evidence of a significant correlation. In 2012, Nombela- Franco et al.5 showed how new onset atrial fibrillation with onset of <24 hours, 0–30 days and 1–30 days after TAVR has an odds ratio for stroke of 2.46, 2.27 and 2.76, respectively. According to another paper, new onset atrial fibrillation in the first year after procedure has an odds ratio for stroke of 4.3.16
In total, chronic atrial fibrillation is already present in 30 % of patients undergoing TAVR; this number increases to 40–50 % when new atrial fibrillation is included.17
Preventing Stroke in Transcatheter Aortic Valve Replacement Patients with Atrial Fibrillation – Chronic and Acute
Chronic Atrial Fibrillation
The optimal strategy for stroke prevention in TAVR patients with preexisting atrial fibrillation is unknown. Patients without pre-existing atrial fibrillation in the PARTNER trial received aspirin 81 mg indefinitely with clopidogrel for three months. The American Association for Thoracic Surgery (AATS)/American College of Cardiology Foundation (ACCF)/Society for Cardiovascular Angiography and Interventions (SCAI)/Society of Thoracic Surgeons (STS) guidelines18 mention that clopidogrel can be continued for 3–6 months. The Canadian Cardiovascular Society (CCS) position statement19 recommends thienopyridine for 1–3 months. For patients with chronic atrial fibrillation, warfarin is substituted for clopidogrel.
Rodés-Cabau et al.17 analysed the evidence behind anticoagulation for patients with atrial fibrillation undergoing TAVR. They found that there was a “lack of uniformity regarding the choice of postprocedural antithrombotic treatment”. This was reflected in a German registry paper of 1,450 patients showing that 7 % of patients received aspirin and clopidogrel monotherapy, 11 % received aspirin or clopidogrel with an oral anticoagulant, 66 % of patients received dual antiplatelet therapy, and 16 % received triple therapy with aspirin, clopidogrel and an oral anticoagulant. The triple therapy was associated with an increased risk of composite of death, stroke, embolism or major bleeding (adjusted odds ratio 1.78, 95 % confidence interval [CI] 1.1– 2.9).20 It therefore seems that for patients with chronic atrial fibrillation who have received TAVR, the use of warfarin with dual antiplatelet therapy is associated with increased risk of bleeding.