MiRNAs Involved in Structural Remodelling
MiRNAs involved in cardiac structural remodelling are miR-21, miR- 26, miR-29b, miR-30, miR-133 and miR-590 (Table 2, Figure 1). These miRNAs regulate genes encoding proteins that are involved in extracellular matrix turnover and pro- or antifibrotic signalling cascades leading to atrial fibrosis as the anatomical substrate for reentry.
miR-21
A first report of miR-21 being involved in profibrotic signalling was published by Thum et al. in 2008.46 In a murine heart failure model, they showed that miR-21 is upregulated mostly in cardiac fibroblasts. By targeting sprouty homologue 1 (Spry1) miR-21 regulated the profibrotic ERK–MAP kinase signalling pathway. In vivo knockdown of miR-attenuates cardiac fibrosis and improves cardiac function.
In a mouse model of myocardial infarction (MI), miR-21 was upregulated leading to repression of the transcription factor phosphatase and tensin homologue (PTEN).47 This blockade results in an upregulation of matrix metalloproteinase 2 (MMP2) and an enhanced matrix turnover suggesting a role for miR-21 in ventricular remodelling.
Finally, miR-21 was detected to be upregulated in left atrial tissue of patients with AF.48 This was associated with reduced expression of Spry1 and increased expression of the profibrotic connective tissue growth factor (CTGF), lysyl oxidase and Rac1-GTPase, resulting in increased collagen content. Additionally, it could be shown in vitro that CTGF and angiotensin II induce an upregulation of miR-21. Finally, Adam and co-workers generated a Rac1-GTPase-knockout mouse and confirmed their previous results in that animal model suggesting a potential mechanism on the profibrotic action of angiotensin II via miR-21.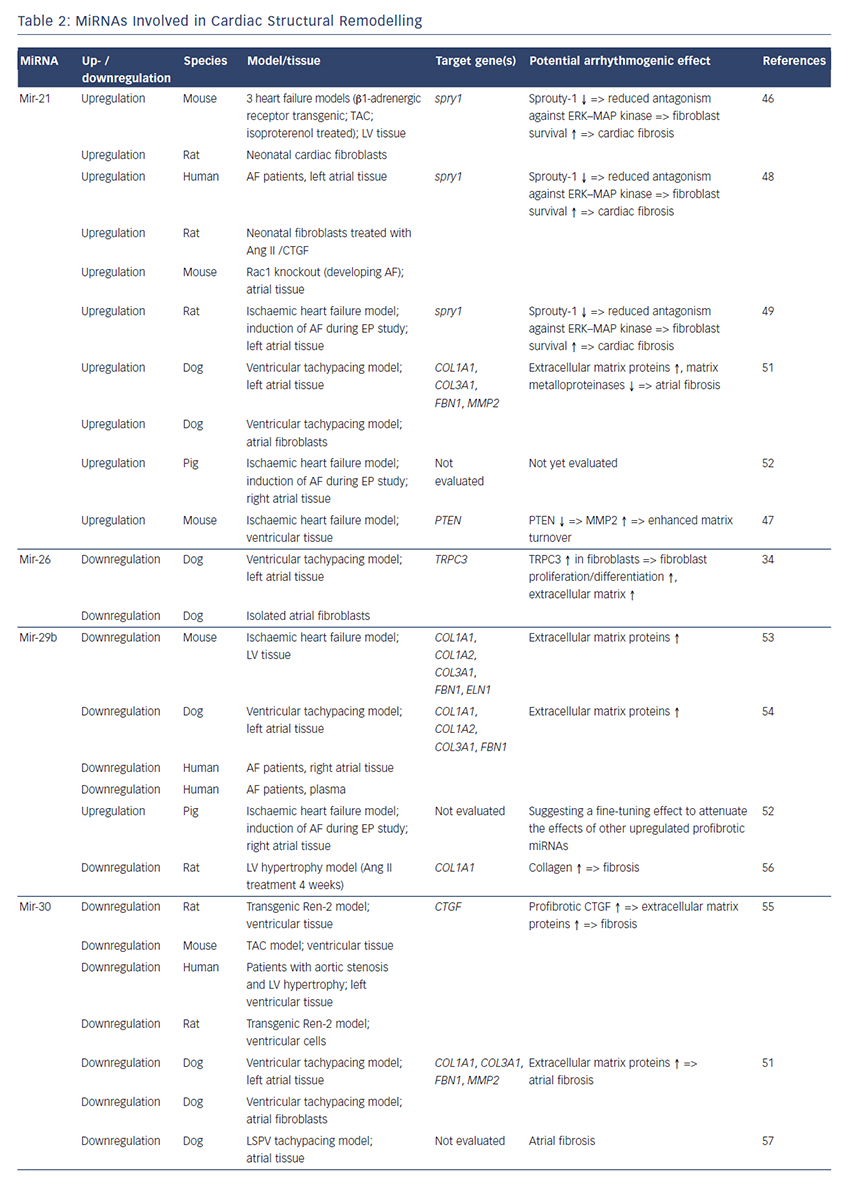
Cardin et al. showed that miR-21 is upregulated in an ischaemic heart failure model in rats.49 Again, Spry1 was confirmed as the miR-21 target gene being downregulated. They injected a miR-21 antagonist directly into the left atria resulting in significant knockdown of miR-21, upregulation of Spry1, improvement of myocardial function, reduction of atrial fibrosis and, finally, reduced AF duration.
Our group evaluated the role of several miRNAs in atrial remodelling in a canine AF model, in which heart failure is induced by ventricular tachypacing, leading to a subsequent atrial remodelling with increased susceptibility for AF.50 We showed that tachycardiomyopathy is associated with a significant upregulation of extracellular matrix proteins (collagen-1, collagen-3 and fibrillin) and a downregulation of MMP2 in left atrial tissue as well as in cardiac fibroblasts.51 These changes were accompanied by increased atrial fibrosis and an upregulation of miR-21. Other miRNAs that are discussed in detail below (miR-29b, miR-30a, miR-133a) were decreased in our model.
Because tachycardiomyopathy is rare in humans we also established an ischaemic heart failure model in pigs to evaluate proarrhythmogenic atrial remodelling processes.52 We induced MI by balloon occlusion of the left anterior descending artery. After 4 weeks of reperfusion, pigs showed a significant heart failure phenotype (left ventricular [LV] pressure increased, LV ejection fraction reduced, LV angiography reduced), significant atrial fibrosis (left and right atria) and were more prone to AF than healthy control pigs (AF inducibility increased, AF burden increased, AF duration/induction increased). In our first analysis, we demonstrated a significant upregulation of miR-21, which accompanied the fibrotic remodelling of the atria.52
In summary, several reports provide consistent evidence regarding a potential role of miR-21 on atrial structural remodelling and AF pathophysiology.
miR-26
Besides its role in electrical remodelling (described above), miR-26 is also reported to be involved in structural remodelling.34 In the canine ventricular tachypacing (VTP) model, miR-26 was downregulated in fibrillating atria causing an upregulation of transient receptor potential cation 3 (TRPC3) channels. These TRPC3 channels have been shown to be expressed in cardiac fibroblasts regulating calcium influx, cell proliferation, extracellular signal-regulated kinase phosphorylation and α-smooth muscle actin protein expression. In vivo blockade of TRPC3 channels prevented the development of an AF substrate in the canine VTP model.
miR-29b
In 2008 van Rooij et al. reported a downregulation of miR-29b in a mouse model of MI that was accompanied with increased fibrosis and an upregulation of extracellular matrix proteins as collagen, fibrillin and elastin.53 They could reproduced their results in vitro by miR-29b overexpression and knockdown, respectively.
Our group evaluated the role of miR-29b in atrial remodelling in the canine VTP model.50 After 24 hours of tachypacing, miR-29b was significantly downregulated in atrial tissue as well as in atrial fibroblasts and remained decreased throughout the time course (up to 2 weeks of ventricular pacing).54 Extracellular matrix proteins (collagen, fibrillin) were upregulated, atrial fibrosis was enhanced and the induced AF duration was significantly prolonged. Lentiviral miR-29b manipulation in fibroblasts mimicked the effects seen in the canine model: overexpression of miR-29b led to a downregulation and miR- 29b knockdown led to an upregulation of extracellular matrix proteins. Interestingly, we confirmed these changes (miR-29b downregulation) in right atrial tissue of patients with AF.
Other miRNAs Involved in Structural Remodelling
Duisters et al. analysed ventricular tissue from rats (hypertensioninduced heart failure model) and mice (transaortic banding [TAC] model), rat cardiac cells and human ventricular biopsy samples (patients suffering from aortic stenosis with LV hypertrophy versus patients who have had coronary artery bypass grafting without LV hypertrophy).55 Their results were consistent over species: miR- 30c and miR-133 were downregulated, whereas their target gene CTGF, a profibrotic mediator, was upregulated. In vitro manipulation achieving miRNA overexpression/knockdown confirmed their results and provided evidence for a potential role of these miRNAs in structural remodelling.
Castoldi et al. injected angiotensin II in rats for 4 weeks resulting in significant hypertrophy and fibrosis.56 They showed a downregulation of miR-133 and miR-29b paralleled by upregulation of collagen-1. All the effects were abolished when rats were treated with irbesartan, suggesting a role for miR-133/miR-29b in angiotensin-II induced cardiac remodelling.
Besides these studies on ventricular hypertrophy, our group identified an atrial downregulation of miR-30 and miR-133 in the canine VTP model accompanied by increased atrial fibrosis.51 This finding was confirmed by Li et al. who induced AF (without heart failure) in dogs by rapid pacing of the left superior pulmonary vein for 5 weeks.57
In an MI model in mice, Wang and co-workers observed a downregulation of miR-499 in the area at risk after ischaemia.58 For a further analysis, they produced a miR-499 transgenic mouse and observed improved cardiac function and reduced collagen content after MI. The catalytic subunits of calcineurin (PPP3CA, PPP3CB) were identified as target genes of miR-499. Further analysis revealed alterations in mitochondrial fission and apoptosis signalling as the potential mechanism underlying the miR-499 actions.
Atrial remodelling was further investigated by Shan et al.37 They administered nicotine in dogs for 30 days resulting in significant increased atrial fibrosis and AF vulnerability compared with healthy dogs. The profibrotic mediator transforming growth factor b 1 (TGF- β1) and the TGF-β receptor-II (TGF-βRII) were significantly upregulated, whereas miR-133 and miR-590 were downregulated in the right atrium. They identified TGF-β1 as the target gene of miR-133 and TGF-βRII as the target gene of miR-590. In vitro manipulation confirmed their in vivo results (up-/downregulation of miR-133/miR-590 resulted in down-/ upregulation of TGF-β1/TGF-βRII/collagen). Finally, they examined right atrial tissue of AF patients and could show that miR-133/miR-590 were downregulated in smokers, suggesting a potential mechanism of the increased risk of AF in smokers.
MiRNAs as Potential Therapeutics
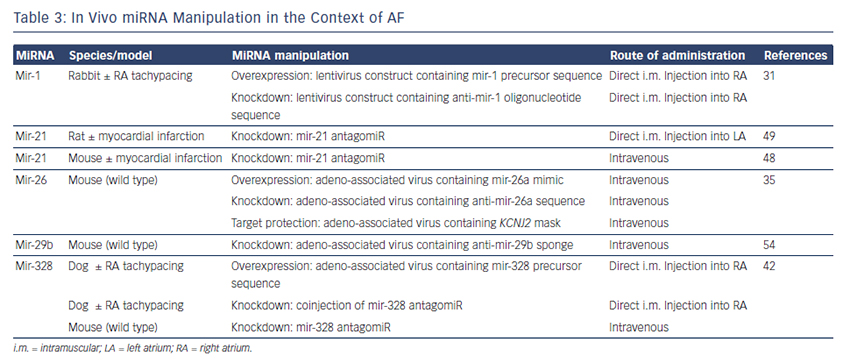
The findings described above demonstrate compelling evidence that miRNAs are powerful factors in AF pathophysiology suggesting in vivo manipulation for AF treatment. So far, several options to agonise or antagonise miRNA effects were developed and successfully evaluated in vivo in AF-related animal models, as described above and summarised in Table 3.31,35,42,48,49,54
Overexpression of a miRNA that is downregulated in disease can be achieved by miRNA mimics. Mimics are synthetic double-stranded RNAs that are incorporated and processed by the cell-like endogenous miRNAs and therefore ‘mimic’ their effects.59 However, mimics are not tissue- or cell-type specific and can therefore create undesirable offtarget effects. This can be avoided by using cardiotropic adenoassociated virus-mediated miRNA transfer that has been shown for the treatment of heart failure in mice60 and cardiac hypertrophy in rats.61
For antagonising a pathological miRNA upregulation, several knockdown approaches are available including anti-miRNA oligonucleotides (antagomiRs)62 or locked nucleic acid,63 miRNA sponges, erasers or masks. AntagomiRs are synthetic oligonucleotides with miRNAcomplementary sequences that bind to endogenous miRNAs competitively inhibit them to bind to their target genes. MiRNA sponges64,65 and erasers62 are sequences of multiple miRNA sequences incorporated into a vector (e.g. a [cardiotropic] virus). While sponges contain only the seed sequence and might therefore inhibit various miRNAs, erasers are complementary to specific miRNAs. MiR-masks, however, are single-stranded oligonucleotides that are complementary to a miRNA target sequence and can therefore specifically block single miRNA–mRNA interactions.35,66
All potential therapeutic interventions are currently based on an intramuscular or systemic application of these agents in vivo. Figure 1 summarises the suspected mechanisms of miRNAs being involved in AF pathophysiology and illustrates potential interventions in order to interrupt the vicious circle of AF begets AF.