Effects of n-3 Fatty Acids on Prevention of Recurrent Atrial Fibrillation
Several trials have been designed to evaluate the effect of n-3 PUFA in the prevention of AF recurrence (see Table 1). In FORWARD (Randomised Trial to Assess Efficacy of PUFA for the Maintenance of Sinus Rhythm in Persistent Atrial Fibrillation), 586 patients with previous symptomatic episodes of AF were randomised to receive 1 g/day 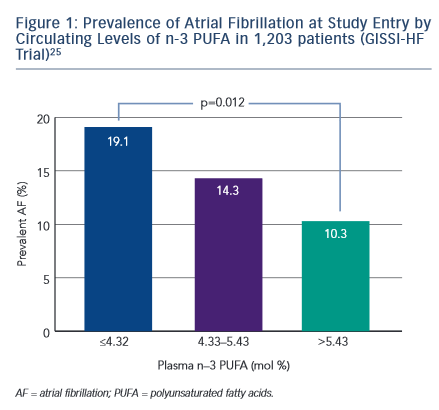 n-3 PUFA or placebo in a double-blind fashion; the primary efficacy endpoint was the time to first recurrence of AF; the follow-up duration was 12 months. At the end of the study, 18.9 % in placebo group and 24.0 % in n-3 PUFA had recurrent AF (HR 1.28 95 % CI 0.90–1.83) and the lack of statistical differences between randomised patientsto placebo or n-3 PUFA were observed for all the other study outcomes (composite of all-cause mortality, non-fatal stroke, non-fatal acute MI, systemic embolism, heart failure development and severe bleeding).24 In a post hoc analysis of the GISSI HF trial (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico Prevenzione), the effect on new episodes of AF of n-3 PUFA daily supplementation was examined in 5,835 patients with heart failure.25 In this trial, fish consumption at study entry was estimated through a self-administered questionnaire and circulating levels of n-3 PUFA were measured in a subgroup of 1,203 patients. More frequent fish consumption with diet was associated, in univariate analysis, with a lower prevalence of AF but after the adjusted analysis fish intake was not associated with AF prevalence while, among patients with measured serum levels of n-3 PUFA, those in the lowest tertile (≤4.32 mmol %) had a twofold higher risk of AF than those in highest tertile (>5.43 mmol %) (OR 1.84; 95 % CI 1.15–2.95; p=0.012) (see Figure 1).25 During the follow-up period of 3.9 years, 15.2 % of the patients allocated to n-3 PUFA and 14.6 % of those randomised to placebo developed AF (unadjusted HR 1.10; 95 % CI 0.96–1.25; p=0.19).
n-3 PUFA or placebo in a double-blind fashion; the primary efficacy endpoint was the time to first recurrence of AF; the follow-up duration was 12 months. At the end of the study, 18.9 % in placebo group and 24.0 % in n-3 PUFA had recurrent AF (HR 1.28 95 % CI 0.90–1.83) and the lack of statistical differences between randomised patientsto placebo or n-3 PUFA were observed for all the other study outcomes (composite of all-cause mortality, non-fatal stroke, non-fatal acute MI, systemic embolism, heart failure development and severe bleeding).24 In a post hoc analysis of the GISSI HF trial (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico Prevenzione), the effect on new episodes of AF of n-3 PUFA daily supplementation was examined in 5,835 patients with heart failure.25 In this trial, fish consumption at study entry was estimated through a self-administered questionnaire and circulating levels of n-3 PUFA were measured in a subgroup of 1,203 patients. More frequent fish consumption with diet was associated, in univariate analysis, with a lower prevalence of AF but after the adjusted analysis fish intake was not associated with AF prevalence while, among patients with measured serum levels of n-3 PUFA, those in the lowest tertile (≤4.32 mmol %) had a twofold higher risk of AF than those in highest tertile (>5.43 mmol %) (OR 1.84; 95 % CI 1.15–2.95; p=0.012) (see Figure 1).25 During the follow-up period of 3.9 years, 15.2 % of the patients allocated to n-3 PUFA and 14.6 % of those randomised to placebo developed AF (unadjusted HR 1.10; 95 % CI 0.96–1.25; p=0.19).
The efficacy of n-3 PUFA supplementation in the prevention of AF was assessed in a randomised trial of 663 outpatients carried out in the US. Patients had symptomatic paroxysmal (542 patients) or persistent AF (121 patients) without structural heart disease and they were treated with 8 g/day of n-3 PUFA for the first 7 days and with 4 g/day thereafter for through 24 weeks. At the end of the study, there were no differences between treatment groups for recurrence of symptomatic AF in both paroxysmal (HR 1.15; 95 % CI 0.90–1.46; p=0.26) and persistent stratum (HR 1.64; 95 % CI 0.92–2.92; p=0.09).26
Several studies have been conducted in the clinical setting of postcardioversion recurrence of AF. The preliminary results of a randomised trial showed that therapy with 1 g/day of n-3 PUFA in 199 patients with recurrent persistent AF was associated with a significantly lower incidence of recurrence compared with placebo at 1 year (40 % versus 72 %; p=0.007).27 These results were not supported by the results of other randomised trials. In the study by Bianconi et al., 204 patients with persistent AF were randomised to receive 3g/day of n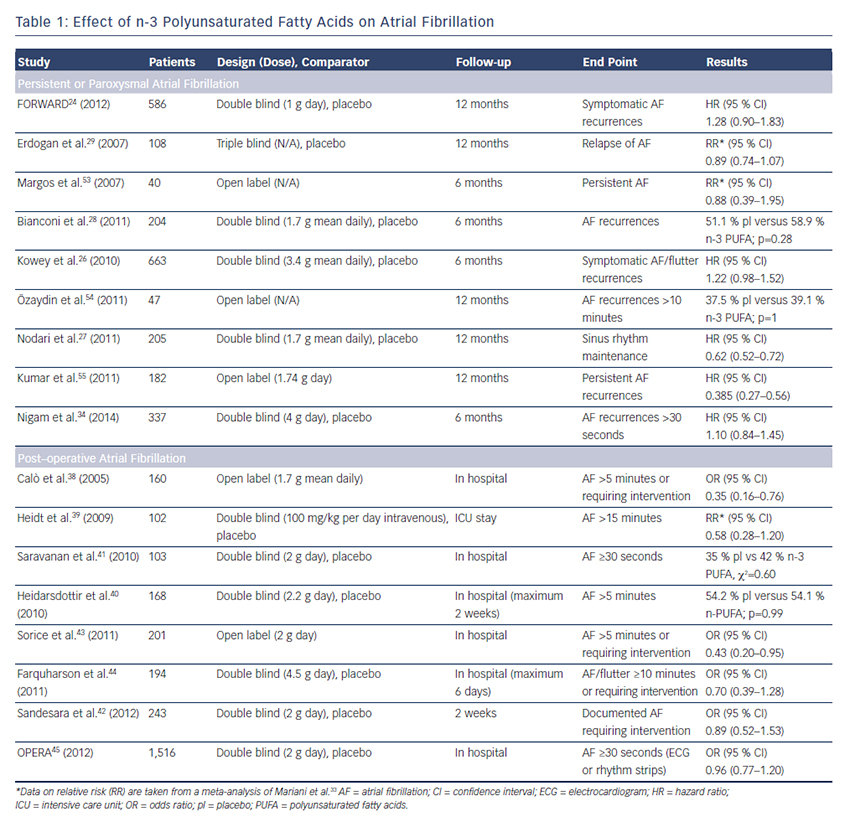 -3 PUFA until electrical cardioversion and 2g/day thereafter for 6 months.28 AF relapsed in 58.9 % of the n-3 PUFA and in 51.1 % of the placebo patients (p=0.28). Similarly, no favourable effects of 4 weeks’ pretreatment with n-3 PUFA on recurrence of AF after cardioversion were shown in 108 patients.29
-3 PUFA until electrical cardioversion and 2g/day thereafter for 6 months.28 AF relapsed in 58.9 % of the n-3 PUFA and in 51.1 % of the placebo patients (p=0.28). Similarly, no favourable effects of 4 weeks’ pretreatment with n-3 PUFA on recurrence of AF after cardioversion were shown in 108 patients.29
Systematic reviews published in the last few years did not provide a definitive answer on the role of n-3 PUFA in prevention of AF probably because the number of patients was relatively small;30–32 in a recent meta-analysis including 16 studies, the analyses were conducted separately for persistent or post-operative AF, eight studies (1,990 patients) evaluated the effect of n-3 PUFA on reverted persistent or paroxysmal AF with a follow-up ranging from 6 to 12 months.33 Treatment had no effect on AF recurrence (RR 0.95; 95 % CI 0.79–1.13) and there were no statistical differences in the overall death rate (RR 0.85; 95 % CI 0.26–2.77). In 2014, a new randomised placebo controlled trial was published: 337 patients with symptomatic paroxysmal or persistent AF, documented within 6 months from enrolment, were randomised to high doses of fish oils (4 g/day) or placebo and followedup on average for 271±129 days. The primary endpoint was time to first AF recurrence lasting at least 30 second and it did not differ between groups (64 % in fish oil group versus 63 % in placebo group; p=0.5). Results were consistent across all pre-specified subgroups such as diabetic, hypertensive, paroxysmal or persistent AF patients, except for a significantly higher AF recurrence for fish oil users with ischaemic heart disease (HR 2.6; 95 % CI 1.1–6.1).34
These data, in combination with those from previous trials, do not recommend the use of n-3 PUFA to prevent recurrent or incident AF (see Table 1).
However, many secondary prevention trials had a short follow-up duration and, for this reason, important benefits from n-3 PUFA could be missed. The Vitamin D and Omega-3 Trial (VITAL) rhythm substudy is examining the impact of the administration of 1 g/day of n-3 PUFA on incident AF 25,875 men and women without cardiovascular disease over 5 years of follow up. It is possible that with a more prolonged duration of treatment a potential benefit could emerge.35