Genetics of PCSK9
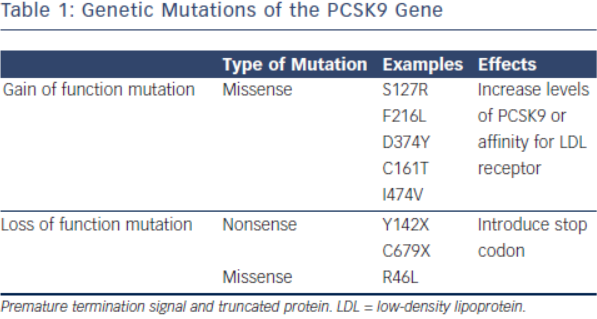 PCSK9 was first discovered as a ninth member of the subtilase subfamily,15 Its function was initially largely unknown until its role in hypercholesterolaemia was realised in a group of French families with autosomal dominant familial hyperlipidaemia (FH). These individuals did not exhibit the usual genetic mutations in the LDLR (LDL receptor) or APOB (apolipoprotein B) genes.16 Instead, the genotype of these FH cohorts revealed multiple missense mutations in the PCSK9 gene resulting in defective intracellular PCSK9 processing (see Table 1). A gain of function mutation results from the defective pro-PCSK9 processing and causes either an over expression of PCSK9 or an increased affinity for the LDL receptor.17 These gain of function mutations have shown to significantly decrease LDL receptors by as much as 35 % and consequently increase serum LDL-C levels.18 Targeting PCSK9 as a therapeutic strategy was conceptualised after loss of function mutations were discovered in both African American and Caucasian populations (see Table 1).19,20 Subjects with low LDL-C in the Dallas Heart Study had PCSK9 sequencing that identified two non-sense mutations in 2 % of the African American cohort resulting in low levels of PCSK9 and a marked reduction of plasma LDL-C of 40 %.21 Similarly, missense mutations were also discovered in the Caucasian population that was associated with 30 % decrease in LDL-C.19 Subjects with loss of function mutations and its association with ischaemic heart disease have been evaluated in a meta-analysis. In multiple groups of subjects with loss of function mutations in the PCSK9 gene, there was a 13 % decrease of LDL-C that was associated with 30 % risk reduction in incidence of ischemic heart disease.20 The higher than expected CVD risk reduction is thought to be from life-long exposure to very low LDL-C levels.
PCSK9 was first discovered as a ninth member of the subtilase subfamily,15 Its function was initially largely unknown until its role in hypercholesterolaemia was realised in a group of French families with autosomal dominant familial hyperlipidaemia (FH). These individuals did not exhibit the usual genetic mutations in the LDLR (LDL receptor) or APOB (apolipoprotein B) genes.16 Instead, the genotype of these FH cohorts revealed multiple missense mutations in the PCSK9 gene resulting in defective intracellular PCSK9 processing (see Table 1). A gain of function mutation results from the defective pro-PCSK9 processing and causes either an over expression of PCSK9 or an increased affinity for the LDL receptor.17 These gain of function mutations have shown to significantly decrease LDL receptors by as much as 35 % and consequently increase serum LDL-C levels.18 Targeting PCSK9 as a therapeutic strategy was conceptualised after loss of function mutations were discovered in both African American and Caucasian populations (see Table 1).19,20 Subjects with low LDL-C in the Dallas Heart Study had PCSK9 sequencing that identified two non-sense mutations in 2 % of the African American cohort resulting in low levels of PCSK9 and a marked reduction of plasma LDL-C of 40 %.21 Similarly, missense mutations were also discovered in the Caucasian population that was associated with 30 % decrease in LDL-C.19 Subjects with loss of function mutations and its association with ischaemic heart disease have been evaluated in a meta-analysis. In multiple groups of subjects with loss of function mutations in the PCSK9 gene, there was a 13 % decrease of LDL-C that was associated with 30 % risk reduction in incidence of ischemic heart disease.20 The higher than expected CVD risk reduction is thought to be from life-long exposure to very low LDL-C levels.

Molecular Mechanism of PCSK9
PCSK9 is a serine protease that belongs to a subfamily of subtilisin proteases responsible for activation or inactivation of other proteins, such as hormones, growth factors and other enzymes. It was initially identified as a seceretory proprotein convertase neural apoptosisregulated convertase-1 (NARC-1) prior to the discovery of its role in cholesterol metabolism.15 PCSK9 proprotein is processed in the endoplasmic reticulum where it undergoes autocatalytic cleavage producing a cleaved, but bound, prodomain and catalytic subunit. The bound prodomain makes the catalytic domain enzymatically inactive, unlike other serine proteases, acting instead as a binding protein when secreted. In the extracellular space, PCSK9 binds to LDL receptor via its catalytic subunit while its C-terminal subunit acts as a chaparone for endocytosis and shuttling the PCSK9-LDLR-LDL complex to lysosomes for degradation.22 In the absence of PCSK9 and in the acidic environment of the clathrin coated endosome, LDLR-LDL complex dissociates and LDLR is recycled back to the cell surface (see Figure 1).23
The PCSK9 gene is located on chromosome 1p32 and its expression is also regulated by intracellular cholesterol, via SREBP-2.24 Synthesis of PCSK9 occurs mostly in the liver, small intestine and kidney and is secreted as a soluble enzyme.25After secretion, 30–40 % of PCSK9 binds to LDL, a process that may inhibit its uptake of LDL by LDLR.26 Low intracellular levels of cholesterol not only stimulate the synthesis of LDL-R but also PCSK9, serving as a counter-regulatory mechanism to maintain intracellular delivery of cholesterol. In the setting of statin, fibrate and ezetimibe use, PCSK9 expression is up-regulated due to low intracellular cholesterol levels.27 The degree of PCSK9 expression correlates with degree of LDL-C levels in subjects treated with statin, attenuating the cholesterol-lowering effects of statin.28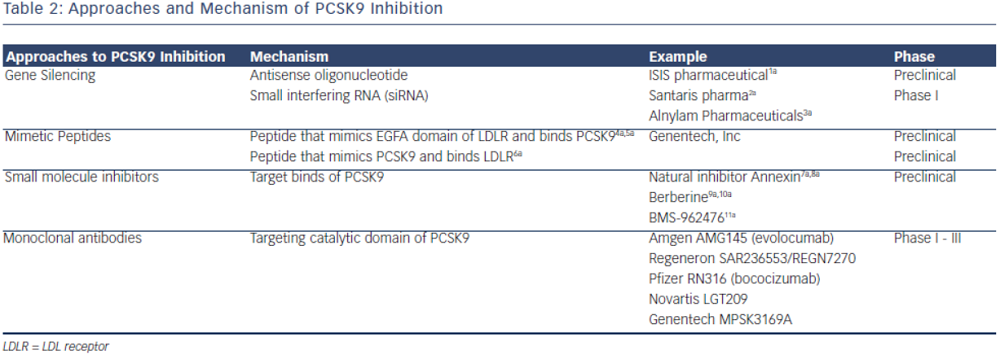 Thus, PCSK9 inhibition is additive to statin therapy and play a synergistic role in its lipid-lowering effects.
Thus, PCSK9 inhibition is additive to statin therapy and play a synergistic role in its lipid-lowering effects.
Inhibition of PCSK9
PCSK9 inhibition is currently achieved by either preventing PCSK9 synthesis or inhibiting the binding of PCSK9 to LDL-R. The most clinically advanced mechanism by which inhibition of PCSK9 has been achieved is through the development of monoclonal antibodies. Other mechanisms, such as mimetic peptides, antisense RNA inhibition and natural binding inhibitors are mostly in preclinical development (see Table 2).