Evolocumab (AMG 145)
Evolocumab, a full human monoclonal antibody, is administered subcutaneously either as every two weeks or every four weeks dosing regimen. Koren at al. showed that escalating doses of evolocumab as a monotherapy in both Q2 week or Q4 week dosing administered subcutaneously to subjects with history of hypercholesterolaemia and low cardiovascular risk (Framingham risk score < 10 %) decreased LDL-C from baseline by up to 48 %.41,42 Stein et al. in the phase II Goal achievement after utilising an anti- PCSK9 antibody in statin intolerant subjects (GAUSS) evaluated the efficacy and safety of evolocumab in patients with statin intolerance.43 Patients receiving escalating doses experienced a significantly reduced LDL-C compared to placebo. At 280 mg dose, LDL-C was reduced by 41 %.43 When escalated to a dose of 420 mg, LDL-C was reduced by 51 %.43 This study was expanded to a phase III trial in subjects with a history of hypercholesterolemia not at previously established National Cholesterol Education Program Adult Treatment Program III (NCEP-ATP III) risk-based goals and intolerant to statins.44 As in the phase II results, any regimen administered significantly reduced LDL-C compared to ezetimibe, with the highest reduction (61 %) seen with evolocumab 420 mg + ezetimibe 10 mg. When administered to subjects who were already taking statin, administration of evolocumab further decreased LDL-C by 63–75 % compared to placebo.45 The use of evolocumab was also studied in subjects with heterozygous FH already on maximized tolerated lipid therapy (stable dose of statin with or without ezetimibe).46,47 As in other studies, the largest additional decrease in LDL-C was seen with dose of 420 mg Q4 week (55 % additional LDL-C reduction).44 In subjects with known CAD or CAD risk equivalent and not at NCEP-ATP III goal of LDL-C ≤ 70 mg/dL, evolocumab was studied as an adjunct to stable doses of statin with or without ezetimibe.48 When administered in escalating doses either as a Q2 week or Q4 week regimen significant reductions in LDL-C occurred. The Q2 week regimen at 140 mg, achieved the highest LDL-C reduction at 12 weeks (64 %). Each dosing group also significantly achieved NCEPATP III treatment goal when compared to placebo (90 % of those in the Q2 week 140 mg group achieved LDL-C ≤ 70 mg/dL).48 An analysis from this study also showed therapy with evolocumab decreased Lp(a) by 23 % compared to placebo.49 As previously discussed, mechanisms by which this effect occurs is unknown.
The use of evolocumab was also studied in homozygous FH, a rare but severely elevated LDL-C, wherein very high CVD risk with possible death in childhood occurs. In the small study by Stein et al., the effect of evolocumab was studied in both LDL-R negative subjects and LDL-R defective patients. Evolocumab significantly reduced LDL-C by 26 % in only the LDL-R defective subjects. In a larger, randomised controlled trial that enrolled homozygous FH, evolocumab consistently decreased LDL-C by 23 %.50
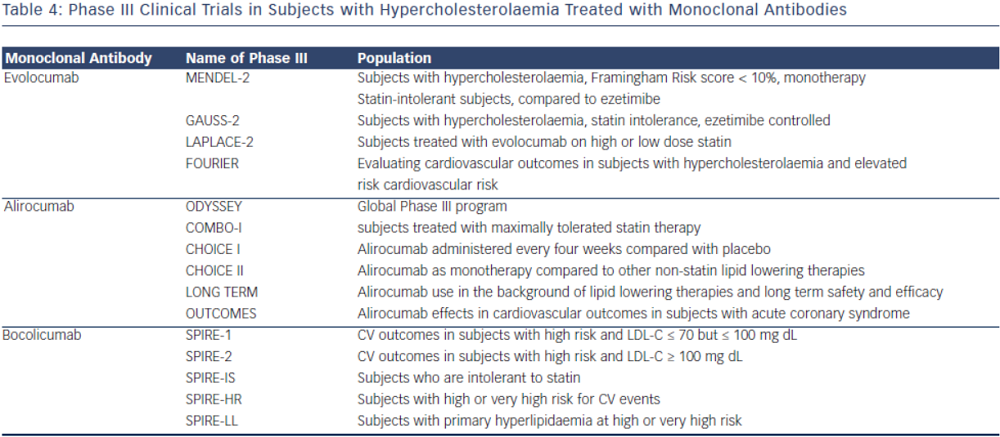
There are currently phase III clinical trials that have shown safety and efficacy in lowering LDL-C with evolocumab. In a 12-week phase III clinical trial, evolocumab use in combination with moderate or high-intensity statin showed significant reduction in LDL-C (up to 75 % reduction when administered every two or four weeks).45 Another phase III study evaluating evolocumab use as a monotherapy also showed significant decrease in LDL-C (57 % more reduction compared to placebo and 40 % more reduction than ezetimibe).42 In a 12-week phase III study evaluating evolocumab use in subjects with intolerance to statin, those treated with evolocumab had significant reduction of LDL-C compared to ezetimibe (53–56 % vs 37–39 % p< 0.001).44 Patients that were previously enrolled in prior phase II studies (GAUSS, RUTHERFORD, LAPLACE-TIMI 57, and MENDEL) were evaluated in the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) trial. This study showed that subjects who continued to take evolocumab for the duration of the year on a monthly dosing regimen, maintained decreased LDL-C levels, whereas those that discontinued the study drug resumed their baseline levels.51 The Further cardiovascular outcomes research with PCSK9 inhibition in Subjects with Elevated CV Risk (FOURNIER) is currently enrolling to evaluate CV outcomes with evolocumab use in subjects already on guideline-based lipid therapy.
 Bococizumab (RN316/PF04950615)
Bococizumab (RN316/PF04950615)
The monoclonal antibody bococizumab has undergone phase I clinical trial and shown that single and escalating intravenous and subcutaneous dose significantly lowered LDL-C by as much as 54 % with 150 mg Q2 week regimen without significant adverse effects.52 Currently, two phase III trials are underway studying bococizumab effects on cardiovascular outcomes in high risk subjects. In one of these outcomes trials, bococizumab use will be evaluated in subjects who are at high risk and have an LDL-C level of 70-100 mg/dL (SPIRE1). In another CV outcomes study, safety and efficacy of bobocizumab will be evaluated in high risk subjects who have higher than LDL 100 mg/ dL, despite high-dose statin or at the highest tolerated dose (SPIRE2) (see Table 4).
Other Targets for PCSK9 Inhibition
Other pharmacologic approaches to PCSK9 inhibition have been proposed; many are still in preclinical development. Direct inhibition of PCSK9 can be attained using small mimetic peptides called adnectins. Mimetic peptides of the PCSK9 binding domain for LDL have been shown to decrease LDL-R degradation.53,54 Mimetic peptides of the binding domain of LDL-R can also bind to PCSK9 and prevent its interaction with LDL-R.53,55 Another approach in PCSK9 inhibition is gene silencing techniques. Antisense oligonucleotides (ASO) are in preclinical development. ASO administration in mice has been shown to increase hepatic LDL-R receptor concentration.56 Antisense RNAs (siRNA) are also being developed to target PCSK9 mRNA, shown in non-human primates to reduce LDL-C by up to 50–70 percent.57 Natural inhibitors, such as annexin A2, a protein expressed in many tissues, inhibit PCSK9 and increase LDL-R.58,59 Berberine, a natural occurring plant alkaloid has been shown to also decrease PCSK9 mRNA expression and increase LDL-R in vitro and animal studies.60,61
Adverse Events
Serious adverse events from monoclonal antibodies targeting PCSK9 are rare. The most common adverse reactions are local injection site reactions. In the GAUSS phase II trial evaluating subjects that have intolerance to statin, myalgias were the most common adverse event but had low incidence overall.43 Alirocumab had similar adverse reactions between placebo and treatment groups in its phase II trials. In a dose escalating study of alirocumab, one of 152 subjects receiving a dose of alirocumab developed cutaneous leukocytoclastic vasculitis that was successfully treated with prednisone.35 Most common adverse reactions that caused discontinuation of medication in these trials were mild injection site reactions (erythema, pruritis, discoloration, haematoma, swelling). For evolocumab, the most common treatment related adverse reaction was not only injection site reaction (pain), but also headache and skin burning sensation.46