Evidence
From cohort B of the Placement of AoRtic TraNscathetER Valves (PARTNER) trial,6 we appreciate that the outcome after TAVI compared with medical therapy in 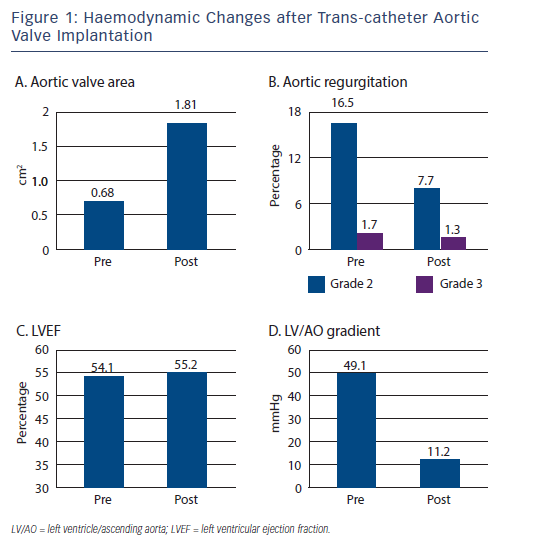 a very high-risk patient group is favourable with significant improved 1-year survival and cardiac symptoms.
a very high-risk patient group is favourable with significant improved 1-year survival and cardiac symptoms.
The Trans-catheter Valve Treatment Sentinel Pilot Registry7 prospectively collected patient data from 4,571 procedures carried out between January 2011 and May 2012, in 137 centres across 10 European countries, using both Sapien XT and CoreValve prostheses. The average age was 81.4+/-7.1 years, logistic euroSCORE 20.2+/-13.3 and New York Heart Association (NYHA) class III or IV was present in 76.9 % of patients. The left ventricular ejection fraction (LVEF) prior to undergoing TAVI was preserved in the majority of cases (mean 54.1+/-13.8 %) (see Figure 1), with only 8.2 % of patients having significantly impaired LVEF <30 %.
 Valve deployment was successful in 96.5 % of patients, although a second valve was required in 2.4 % of cases, and surgical conversion occurred in 4.3 %. Analysis of in-hospital mortality demonstrated a sizeable difference between approaches - total mortality reached 7.4 %, the transfemoral approach was associated with a notably lower risk at 5.9 %, while transapical (12.8 %) and trans-subclavian and other approaches (9.7 %) were associated with adverse outcomes (most likely as a consequence of differing clinical characteristics). A significant difference in permanent pacing requirements was also observed between devices used - the CoreValve resulting in 23.4 % versus 6 % for Sapien XT valves. Similarly, a lower rate of aortic regurgitation (AR) was associated with use of Sapien XT valve (3.8 % versus 6.7 % in the CoreValve valve for both grade 2 and 3 AR). The duration of hospital stay varied between countries (mean 9.3+/-8.1 days), being more prolonged if general anaesthesia had been administered (10.2+/-8.7 days versus 7.9+/-6.1 days) and if a trans-apical or another surgical approach had been used (43.8 % and 39.5 %
Valve deployment was successful in 96.5 % of patients, although a second valve was required in 2.4 % of cases, and surgical conversion occurred in 4.3 %. Analysis of in-hospital mortality demonstrated a sizeable difference between approaches - total mortality reached 7.4 %, the transfemoral approach was associated with a notably lower risk at 5.9 %, while transapical (12.8 %) and trans-subclavian and other approaches (9.7 %) were associated with adverse outcomes (most likely as a consequence of differing clinical characteristics). A significant difference in permanent pacing requirements was also observed between devices used - the CoreValve resulting in 23.4 % versus 6 % for Sapien XT valves. Similarly, a lower rate of aortic regurgitation (AR) was associated with use of Sapien XT valve (3.8 % versus 6.7 % in the CoreValve valve for both grade 2 and 3 AR). The duration of hospital stay varied between countries (mean 9.3+/-8.1 days), being more prolonged if general anaesthesia had been administered (10.2+/-8.7 days versus 7.9+/-6.1 days) and if a trans-apical or another surgical approach had been used (43.8 % and 39.5 % 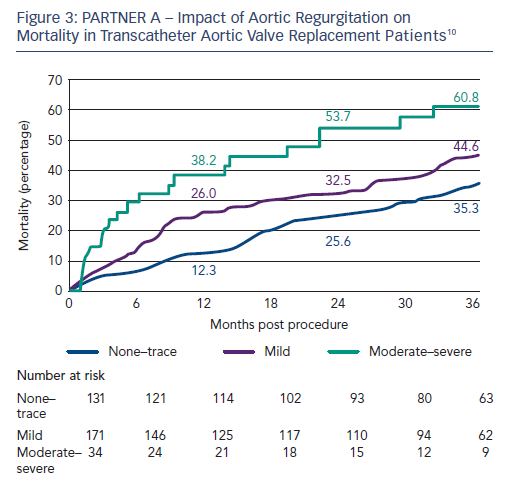 stayed in hospital >10 days versus 22.0 % of patients treated trans-femorally).
stayed in hospital >10 days versus 22.0 % of patients treated trans-femorally).
This registry has highlighted that, in Europe, TAVI is still reserved for older patients, and is rarely used in patients under the age of 70 (where special circumstances and/or prohibitive comorbidities may exist).
 The 1-year outcomes of the SAPIEN Aortic Bioprosthesis European Outcome-XT (SOURCE) study were presented on behalf of the investigators by Dr Stephan Windecker during the European Association of Percutaneous Cardiovascular Interventions (EuroPCR) congress in 2013.8 This is a prospective, observational registry to assess the effectiveness and major adverse events in all patients implanted with a Sapien XT valve (Edwards Lifesciences), via trans-femoral or transapical access and worth noting that the patients included had a slightly lower mean euroSCORE when compared with the patients enrolled in the earlier
The 1-year outcomes of the SAPIEN Aortic Bioprosthesis European Outcome-XT (SOURCE) study were presented on behalf of the investigators by Dr Stephan Windecker during the European Association of Percutaneous Cardiovascular Interventions (EuroPCR) congress in 2013.8 This is a prospective, observational registry to assess the effectiveness and major adverse events in all patients implanted with a Sapien XT valve (Edwards Lifesciences), via trans-femoral or transapical access and worth noting that the patients included had a slightly lower mean euroSCORE when compared with the patients enrolled in the earlier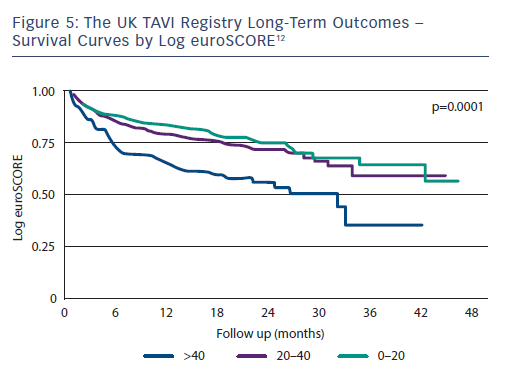 SOURCE registry of recipients of the predecessor Sapien valve. From 93 centres in 17 countries, 2,688 patients were included in the study. Procedural complication risks (within 48 hours) were as follows: death 2.3 %, stroke 2.2 %, cardiac tamponade 0.9 %, permanent pacemaker implantation 5.7 %, major/life-threatening bleeding 10.8 % and vascular access-related complications 11 %.
SOURCE registry of recipients of the predecessor Sapien valve. From 93 centres in 17 countries, 2,688 patients were included in the study. Procedural complication risks (within 48 hours) were as follows: death 2.3 %, stroke 2.2 %, cardiac tamponade 0.9 %, permanent pacemaker implantation 5.7 %, major/life-threatening bleeding 10.8 % and vascular access-related complications 11 %.
Again, a significant disparity between vascular access and mortality was noted, with the highest 1-year survival in the trans-femorally treated patients (85 %), and other routes carrying apparent increased risk (trans-apical and trans-aortic 1-year survival 72.8 % and 73.9 %, respectively). However, the patients requiring an alternative to femoral access are likely to have comorbidities that make this route unsafe (severe peripheral vascular disease with associated cerebrovascular and coronary artery disease), and may therefore be considered inherently higher-risk patients.
The all-cause mortality at 1 year was 19.5 %, and cardiac mortality 10.8 %, which is encouraging in showing a downward trend in mortality rates after TAVI, and also demonstrating a lower incidence of para-valvular regurgitation (PAR) at 1 year than previously reported (6.2 % versus 10.5 % in the PARTNER B trial).6 In multivariate analyses, only porcelain aorta, liver disease, renal impairment, significant tricuspid regurgitation and coronary artery disease (not PAR) emerged as predictors of mortality.
Substantial symptomatic benefit is achieved one year after TAVI with the proportion of patients in NYHA class III or IV reducing from 75.3 % to 9.7 %. Similarly, quality of life (assessed by the EuroQoL 5D [EQ- 5D] scale) improved from 49.3 to 69.5, and the proportion of patients with angina reduced from 45 % at baseline to 20.6 %.9 Women fared better than men with 90.6 % versus 87.6 % free from cardiac events, and 82.5 % versus 77.9 % freedom from all-cause events at 1 year.
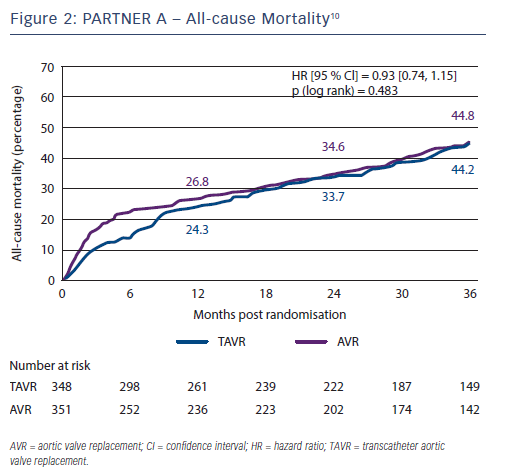
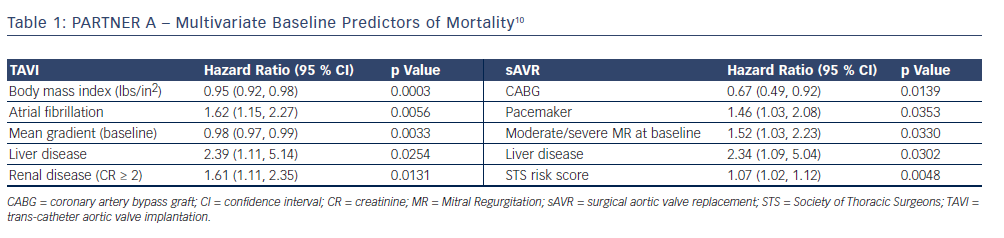
The 3-year outcome of the PARTNER cohort A trial in 699 operable high-risk patients with severe aortic stenosis after TAVI or sAVR was presented by Dr Vinod Thourani at the American College of Cardiology Scientific Session Summit in March 201310 and revealed similar all-cause mortality (44.2 % versus 44.8 %, respectively; p=0.483) and stroke rates (8.2 % versus 9.3 %; p=0.763) between the two procedures (see Figure 2); therefore, very importantly confirming TAVI as a non-inferior treatment option in this high-risk group. Baseline outcome predictors for the two procedures differed when assessed with multivariate analysis (see Table 1), and complications are shown in Table 2. There was a higher burden of moderate or severe PAR after TAVI than sAVR at 1, 2 and 3 years, which conferred a higher risk of mortality - this adverse effect was demonstrable even in patients with mild PAR (see Figure 3).
 Repositionable percutaneous replacement of a stenotic aortic valve through implantation of the Lotus Valve System (REPRISE II) is a prospective registry of 120 patients evaluating the efficacy and safety of the Lotus Valve System, a differentiated second-generation TAVI technology which consists of a pre-loaded, stent-mounted tissue valve prosthesis and catheter for delivery and placement of the valve. Initial data presented by Dr Ian Meredith at EuroPCR in 2013,11 demonstrated low 30-day mortality and stroke rates (1.7 % and 3.4 %, respectively) with a further 5.2 % suffering a non-disabling stroke. There was minimal aortic regurgitation and mean aortic gradient improved significantly from 47.5+/-17.2 mmHg to 11.3+/-5.2 mmHg at 30 days. There was, however, a need for permanent pacing in 29.3 % of patients.
Repositionable percutaneous replacement of a stenotic aortic valve through implantation of the Lotus Valve System (REPRISE II) is a prospective registry of 120 patients evaluating the efficacy and safety of the Lotus Valve System, a differentiated second-generation TAVI technology which consists of a pre-loaded, stent-mounted tissue valve prosthesis and catheter for delivery and placement of the valve. Initial data presented by Dr Ian Meredith at EuroPCR in 2013,11 demonstrated low 30-day mortality and stroke rates (1.7 % and 3.4 %, respectively) with a further 5.2 % suffering a non-disabling stroke. There was minimal aortic regurgitation and mean aortic gradient improved significantly from 47.5+/-17.2 mmHg to 11.3+/-5.2 mmHg at 30 days. There was, however, a need for permanent pacing in 29.3 % of patients.
The UK TAVI registry12 followed the outcomes of all patients with severe symptomatic aortic stenosis undergoing TAVI with the CoreValve and Edwards Sapien 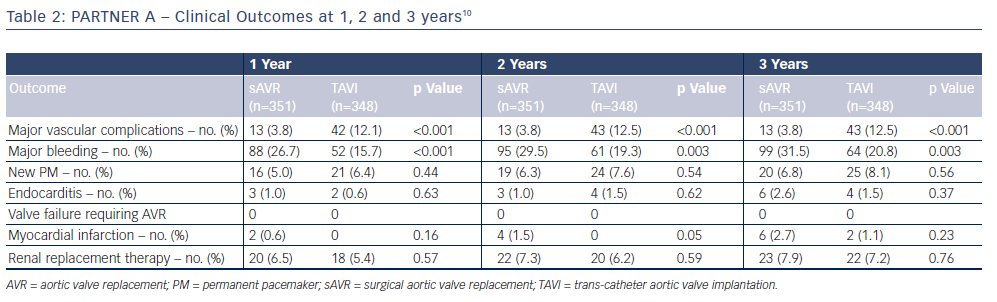 valves in England and Wales between the first implant in January 2007 to December 2009 (877 implants in 870 patients). Here, 69 % of cases were carried out via the trans-femoral route, whereas patients with peripheral vascular disease, coronary artery disease, prior cardiac surgery, renal dysfunction, and NYHA class III or IV were more likely to have the procedure carried out through alternative access. The median age was 81.9+/-7.1 years and median logistic euroSCORE 18.5 %. Eight cases were unsuccessful (0.9 %) and emergency conversion to sAVR occurred in 6 patients (0.7 %). Again, there was a higher need for permanent pacing following CoreValve implantation when compared with Sapien (24.4 % versus 7.4 %; p<0.0001). Survival at 30 days, 1 year and 2 years was 92.9 %, 78.6 % and 73.7 %, respectively, with no statistical difference between the Sapien and CoreValve cohort. However, a maintained survival benefit was noted with the trans-femoral route compared with alternative access (81.5 % versus 72.3 % at 1 year; p=0.002; 77.5 % versus 65.3 % at 2 years; p=value <0.001). The UK TAVI investigators also noted that these survival rates are consistent with outcomes in the UK surgical database for octogenarians undergoing sAVR or sAVR with coronary artery bypass grafting.13
valves in England and Wales between the first implant in January 2007 to December 2009 (877 implants in 870 patients). Here, 69 % of cases were carried out via the trans-femoral route, whereas patients with peripheral vascular disease, coronary artery disease, prior cardiac surgery, renal dysfunction, and NYHA class III or IV were more likely to have the procedure carried out through alternative access. The median age was 81.9+/-7.1 years and median logistic euroSCORE 18.5 %. Eight cases were unsuccessful (0.9 %) and emergency conversion to sAVR occurred in 6 patients (0.7 %). Again, there was a higher need for permanent pacing following CoreValve implantation when compared with Sapien (24.4 % versus 7.4 %; p<0.0001). Survival at 30 days, 1 year and 2 years was 92.9 %, 78.6 % and 73.7 %, respectively, with no statistical difference between the Sapien and CoreValve cohort. However, a maintained survival benefit was noted with the trans-femoral route compared with alternative access (81.5 % versus 72.3 % at 1 year; p=0.002; 77.5 % versus 65.3 % at 2 years; p=value <0.001). The UK TAVI investigators also noted that these survival rates are consistent with outcomes in the UK surgical database for octogenarians undergoing sAVR or sAVR with coronary artery bypass grafting.13
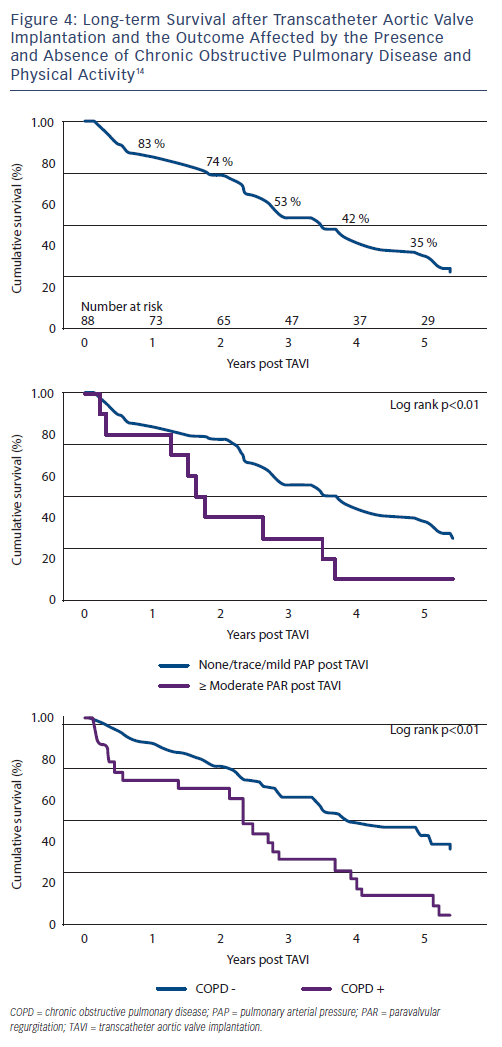
A 5-year outcome analysis of the first 111 TAVIs performed between 2005 and 2007 using the Cribier-Edwards or Edwards SAPIEN valve in a single centre in 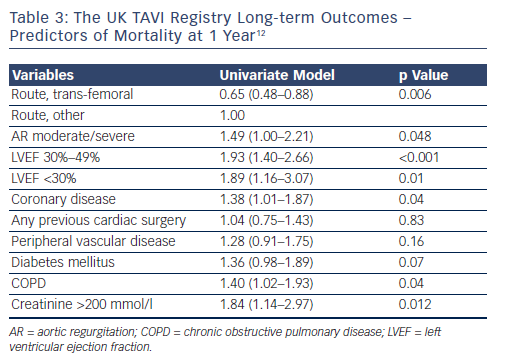 Canada14, reported unsuccessful implantation in 8 cases and 15 deaths occurred within 30 days. Of the remaining 88, 84 (median age 83+/-7 years) were followed up with a median survival time of 3.4 years (survival rates at 1 to 5 years were 83 %, 74 %, 53 %, 42 % and 35 %, respectively). The presence of chronic obstructive pulmonary disease (COPD) and at least moderate PAR post-TAVI were associated with a higher risk of mortality (hazard ratio 2.17 [95 % confidence interval 1.18 to 3.70] and 2.98 [95 % confidence interval 1.44 to 6.17] respectively) (Figure 4). Post-procedure, 25 % of patients had no PAR, 63 % had mild PAR and 11.4 % had at least moderate PAR.
Canada14, reported unsuccessful implantation in 8 cases and 15 deaths occurred within 30 days. Of the remaining 88, 84 (median age 83+/-7 years) were followed up with a median survival time of 3.4 years (survival rates at 1 to 5 years were 83 %, 74 %, 53 %, 42 % and 35 %, respectively). The presence of chronic obstructive pulmonary disease (COPD) and at least moderate PAR post-TAVI were associated with a higher risk of mortality (hazard ratio 2.17 [95 % confidence interval 1.18 to 3.70] and 2.98 [95 % confidence interval 1.44 to 6.17] respectively) (Figure 4). Post-procedure, 25 % of patients had no PAR, 63 % had mild PAR and 11.4 % had at least moderate PAR.
Predictors of mortality at 1 year are listed in Table 3, and the survival curves according to logistic euroSCORE plotted in Figure 5. Although there is significantly worse prognosis for the patients with euroSCOREs >40 after 30 days, no survival difference emerged among lower-risk cohorts.
Haemodynamic benefits were impressive and sustained. There was a significant improvement in aortic valve area from 0.62+/-0.17 cm2 to 1.67+/-0.41 cm2, with persistence of this benefit 5 years post procedure (1.40+/-0.25 cm2). The same trend was seen with mean aortic valve gradient from 46+/-18 mmHg to 10+/-4.5 mmHg pre and immediately post procedure, to 11.8+/-5.7 mmHg at 5 years. At 4 years, there were no features of prosthetic valve failure, although three patients (3.4 %) developed moderate trans-valvular regurgitation and/or stenosis at 5 years.
Interestingly, nearly half of the patients (48 %) had concomitant moderate/severe mitral regurgitation (MR) at the time of TAVI which improved to no/mild MR in 57 % of cases post-operatively.