Procedural Details
A more thorough description of percutaneous PVL closure techniques can be found elsewhere.14 Briefly, percutaneous mitral PVL closure can be performed via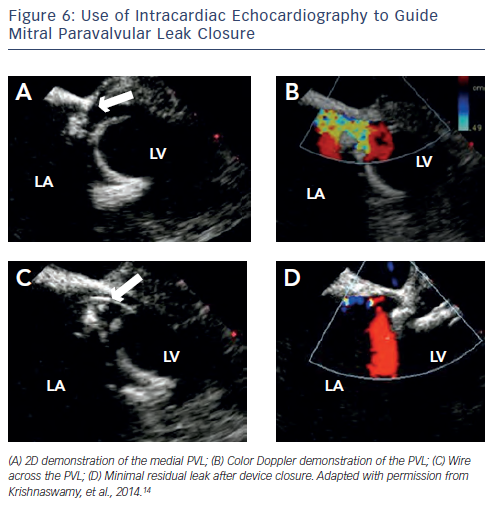 femoral vein access and transseptal puncture, left ventricular (LV) apical access, or retrograde via the femoral artery. Closure of aortic PVL is most effectively accomplished retrograde via the femoral artery. Tricuspid PVL can be accomplished via the jugular vein. The choice of access site should be decided on the basis of PVL location, support required for delivery of the bulky closure devices, and presence of other mechanical prostheses that may interfere with wire-snaring/externalisation. As we perform the majority of our PVL closure procedures without general anaesthesia or endotracheal intubation, minimising TOE-probe dwell time is essential for patient comfort. We therefore rely on ICE (see Figure 6) for transseptal puncture and when possible for PVL guidance (see Figure 6). We have also demonstrated previously the feasibility of integrating computed tomography (CT) data onto the realtime fluoroscopic image using a C-arm based CT acquisition (Syngo DynaCT, Siemens Healthcare, Forchheim, Germany) (see Figure 7).15 These techniques allow us to place the TOE probe only after the device is in place to confirm adequate PVL closure.
femoral vein access and transseptal puncture, left ventricular (LV) apical access, or retrograde via the femoral artery. Closure of aortic PVL is most effectively accomplished retrograde via the femoral artery. Tricuspid PVL can be accomplished via the jugular vein. The choice of access site should be decided on the basis of PVL location, support required for delivery of the bulky closure devices, and presence of other mechanical prostheses that may interfere with wire-snaring/externalisation. As we perform the majority of our PVL closure procedures without general anaesthesia or endotracheal intubation, minimising TOE-probe dwell time is essential for patient comfort. We therefore rely on ICE (see Figure 6) for transseptal puncture and when possible for PVL guidance (see Figure 6). We have also demonstrated previously the feasibility of integrating computed tomography (CT) data onto the realtime fluoroscopic image using a C-arm based CT acquisition (Syngo DynaCT, Siemens Healthcare, Forchheim, Germany) (see Figure 7).15 These techniques allow us to place the TOE probe only after the device is in place to confirm adequate PVL closure.
Choice of Device
 There are no devices created specifically for percutaneous PVL closure; those that are used are created for other applications, such as closure of septal defects or vascular plugs (see Figure 8). An important part of planning the percutaneous PVL closure is understanding the shape and size of the defect. We find that given the crescentic shape of PVLs, and the generally round shape of the devices, multiple devices are often necessary to adequately close the leak. It is important, especially in the setting of mechanical valve replacements, to be watchful of impingement on the prosthetic valve apparatus.
There are no devices created specifically for percutaneous PVL closure; those that are used are created for other applications, such as closure of septal defects or vascular plugs (see Figure 8). An important part of planning the percutaneous PVL closure is understanding the shape and size of the defect. We find that given the crescentic shape of PVLs, and the generally round shape of the devices, multiple devices are often necessary to adequately close the leak. It is important, especially in the setting of mechanical valve replacements, to be watchful of impingement on the prosthetic valve apparatus.
We most often use the Amplatzer™ Vascular Plug II (AVP II; St. Jude Medical, Minnesota, US), which consists of a nitinol cylinder with a nitinol disc on either side. The AVP I is a single cylinder design, making it less stable and effective in our experience, and the AVP III is not available in the US. We find that the use of atrial septal defect (ASD) occlusion devices is often complicated by the large discs that can interfere with the prosthetic valve, and ventricular septal defect (VSD) closure devices are quite stiff and often result in worsening haemolysis. Patent ductus arteriosus (PDA) occluders are available in limited sizes, but are sometimes helpful when the AVP II discs interfere with valve leaflet motion.
Complications of Paravalvular Leak Closure
Percutaneous PVL closure in trained centres has demonstrated good efficacy and safety. It is important in 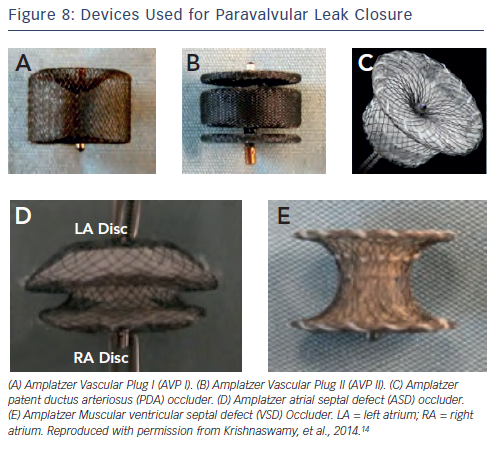 the performance of these procedures, to be aware of the potential complications in order to plan accordingly.
the performance of these procedures, to be aware of the potential complications in order to plan accordingly.
The atrioventricular node is situated at the junction of the interatrial septum and the interventricular septum. Therefore, closure of PVLs close to this position can be complicated by complete heart block at the time of device implantation. Pre-emptive temporary pacemaker placement may be reasonable in such cases.
Care must be taken to observe mechanical valve function fluoroscopically and by echocardiography to assure that the PVL closure device does not produce valve dysfunction prior to release. In our experience, it is rare that PVL closure cannot be completed due to valve interference, though attempt at different devices and sizes may be necessary for a successful procedure.
In patients for aortic PVL, proximity to the native right and left coronary arteries should be considered, along with the relative size of the aortic sinus. In some cases, it is helpful to take an aortic root angiogram to further define this space prior to the placement of a PVL closure device.
Embolisation of the occluder devices has been reported in <1 % to 5 % of large series’.10,13 A sudden change in symptoms or atrial or ventricular ectopy may be early clues to device embolisation. When it does occur, percutaneous retrieval is usually performed successfully using snare devices and/or bioptomes. Alternatively, if the device is permanently lodged within the LV without significant risk for further mobilisation, consideration can be given to leaving it in place with monitoring by CT or echocardiography during follow-up.10 Echocardiographic follow-up is also important to reassess device placement and integrity of the valve itself as further valve dehiscence over time has been reported.