Chordae Tendineae/Papillary Muscles
The chordae tendineae originate from the papillary muscles and attach to the mitral leaflets, transmitting ventricular contractions.3 The primary and secondary chordae maintain leaflet apposition and facilitate valve closure, whereas the tertiary chordae help to maintain ventricular geometry.5 Abnormalities of the chordae tendineae typically include rupture and presence of abnormally long or short chordae.1 Papillary dysfunction can occur with fibrosis, ischaemia, or rupture through infarction or trauma. Elongated degenerated papillary muscles can induce prolapse and retracted papillary muscles can make cords vulnerable to disruption.
Chordal Implants/Cinching
Chordal implants are synthetic chords or sutures that can be used to correct leaflet prolapse usually due to ruptured or torn chordae. The implants are attached to the free leaflet margins and anchored to the papillary muscles or LV myocardium. The chords can be delivered either via a transseptal or transapical approach. V-Chordal (Valtech Cardio LTD, Or-Yehuda, Israel), originally designed for an off-pump transatrial approach, allows for chordal implantation with dynamic modulation, able to adjust chordal length under physiological conditions to optimise leaflet coaptation. The device is initially screwed (sutureless) into 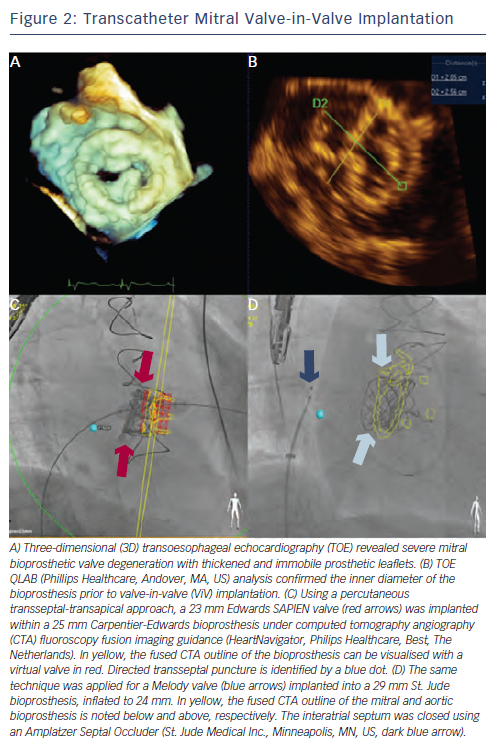 the papillary muscles; then the chordal length is adjusted by turning a nob; they are subsequently secured to the leaflet margin. FIM trial of seven patients revealed complete procedural success with 100 % chronic success at two years.23 The newer transseptal system has a cinching device to secure the chord with two chords allowed for each implant. Preclinical trials are underway.
the papillary muscles; then the chordal length is adjusted by turning a nob; they are subsequently secured to the leaflet margin. FIM trial of seven patients revealed complete procedural success with 100 % chronic success at two years.23 The newer transseptal system has a cinching device to secure the chord with two chords allowed for each implant. Preclinical trials are underway.
The NeoChord (NeoChord, Eden Prairie, MN, US) system allows for transapical deployment with fibre optic confirmation of leaflet grasping.24 This device is mentioned due to its off-pump application and potential for percutaneous delivery. Transapical Artificial Chordae Tendineae (TACT) trial enrolled 30 patients with severe MR and isolated posterior leaflet prolapse who underwent placement of at least one artificial NeoChord.25 Acute procedural success was noted in 87 % of patients with 65 % achieving MR reduction to Ôëñ2+ at 30 days. The implantation of multiple chords (2-4 per patient) and fixation to the posterolateral wall translated into higher success rates. All patients requiring subsequent surgery were able to undergo successful repair. NeoChord recently achieved CE mark. Overall, the concern with chordal implants is that aggressive therapy may lead to leaflet restriction and residual MR. Furthermore, the presence of intracardiac material may also predispose the patient to thrombus formation.
Mitral Valve Replacement
Despite the desire to repair rather than to replace the mitral valve, TMVT are currently limited. With the ability to preserve the mitral apparatus, TMVR may be an alternative to repair technologies when suboptimal results are achieved or even a more suitable approach. Unlike the aortic valve and transcatheter aortic valve replacement, the complexities of the mitral apparatus make TMVR a challenge. Concerns for TMVR include the presence of larger annular sizes and asymmetric MA anatomy, the need for appropriate valve anchoring within the MA, and the potential for developing left ventricular outflow tract (LVOT) obstruction and paravalvular regurgitation. Durability issues dealing with stent fracture, tissue erosion and degeneration require evaluation. Multiple TMVR devices that have been developed are in preclinical testing or FIM trials.
The CardiAQ Prosthesis (CardiAQ Valve Technologies Inc., Irvine, CA, US) is a porcine pericardial, trileaflet valve on a nitinol frame that is transseptally delivered.13 It has two sets of 12 anchors that secure the device within the MA and preserve the chordae and papillary muscles. It was the first valve to achieve successful FIM in 2012 for severe MR. This patient died on day three due to multisystem organ failure with autopsy not revealing evidence of prosthetic issues. The Cardiovalve® prosthesis (Valtech Cardio Ltd) is also transfemorally delivered through a transeptal approach.26 The device has separate fixation and sealing, where the sealing skirt is first implanted followed by the valve. The novel fixation design around both the anterior and posterior leaflets is able to generate >50 N of anchoring force. Preclinical trials are promising and ongoing.
On the other hand, the Tiara prosthesis (Neovasc Inc., Vancouver, BC, Canada) is a trileaflet porcine pericardial valve fixed on a self-expanding frame that is delivered transapically.14,15,27 The frame/valvular orifice is D-shaped to mimic the natural shape of the mitral orifice; the flat side of the D is positioned toward the aortic-mitral continuity to prevent interruption with the aortic valve and LVOT. Its atrial lip engages the left atrium and three ventricular anchors secure the valve in place during systole. The ventricular portion of the device has a covered skirt to reduce risk of paravalvular regurgitation. Tiara is repositionable and retrievable, and awaiting FIM later this year. The Lutter prosthesis (Tendyne Medical Inc., Baltimore, MD, US) is a trileaflet bovine pericardial valve fixed on a self-expanding frame.27,28 The device, like the Tiara, is delivered transapically and has an atrial fixation system or flat ring that is connected at a 45, 90 or 110 degree angle (three different iterations of the device) to the tubular ventricular portion of the stent. In addition, there is a ventricular fixation system where neochords are attached to the apex to secure the valve in place and a covered skirt to reduce paravalvular regurgitation. Preclinical trials are underway.
 Next, the Endovalve™ prosthesis (Micro Interventional Devices Inc.™, Bethlehem, PA, US) is a trileaflet bovine pericardial valve that was initially delivered transatrially via a right thoracotomy.29 The device is made of a foldable nitinol frame that attaches to the native valve through three anchors or grippers, grasping the native leaflets and MA; a Dacron skirt covers the valve. Recent concern over valve fixation has stimulated valve redesign to create an active fixation system where the device is delivered transapically and anchors are attached to a novel transapical closure device, Permaseal™ (Micro Interventional Devices Inc.). Preclinical trials of the newer generation device are still pending.
Next, the Endovalve™ prosthesis (Micro Interventional Devices Inc.™, Bethlehem, PA, US) is a trileaflet bovine pericardial valve that was initially delivered transatrially via a right thoracotomy.29 The device is made of a foldable nitinol frame that attaches to the native valve through three anchors or grippers, grasping the native leaflets and MA; a Dacron skirt covers the valve. Recent concern over valve fixation has stimulated valve redesign to create an active fixation system where the device is delivered transapically and anchors are attached to a novel transapical closure device, Permaseal™ (Micro Interventional Devices Inc.). Preclinical trials of the newer generation device are still pending.
Finally, the Ventor Embracer prosthesis (Medtronic Inc., Minneapolis, MN, US) is a trileaflet bovine pericardial valve on a self-expanding nitinol frame.30 The device consists of two components: a large inflow portion that seals to the atrial surface of the MA, and a very short LV projection to avoid subannular and LVOT interference. Support arms engage the A2P2 components of the mitral valve, securing the valve in place and creating tension on the atrial portion to improve apposition and reduce paravalvular regurgitation. The device is delivered through a transatrial approach. Preclinical trials are also underway.
Valve-in-Valve/Valve-in-Ring Therapy
Although the TMVR technologies remain in its early clinical stages, current transcatheter valves are being implanted successfully within surgical mitral platforms such as degenerative bioprostheses and complete annuloplasty rings. For patients with degenerative bioprostheses or failed repair, reoperative surgical valve replacement often carries a high mortality risk, especially in older patients with multiple co-morbidities. Transcatheter valve-in-valve (ViV) or valve-in-ring (ViR) implantation is a less invasive option and can be utilised as an alternative to open surgery in the high-risk or inoperable patient.31-33
In the mitral position, the transcatheter valves that are currently utilised 'off-labelÔÇÖ are the Edwards Sapien (Edwards Lifesciences, Irvine, CA, US) and the Melody┬« (Medtronic Inc.) valves. The Edwards Sapien valve is a bovine pericardial valve sutured onto a stainless steel balloon-expandable stent that is Food and Drug Administration (FDA) approved and CE marked for transcatheter aortic valve replacement. The Melody valve is that of a bovine jugular valved vein, which is sutured onto a platinum-iridium stent. It is designed for dysfunctional right ventricular outflow tract conduits. To date, the results of seven Melody ViV implantations within a high-pressure, left-sided haemodynamic environment (one mitral, six aortic) revealed complete freedom from regurgitation and an 86 % freedom from significant stenosis at one-year follow-up.24
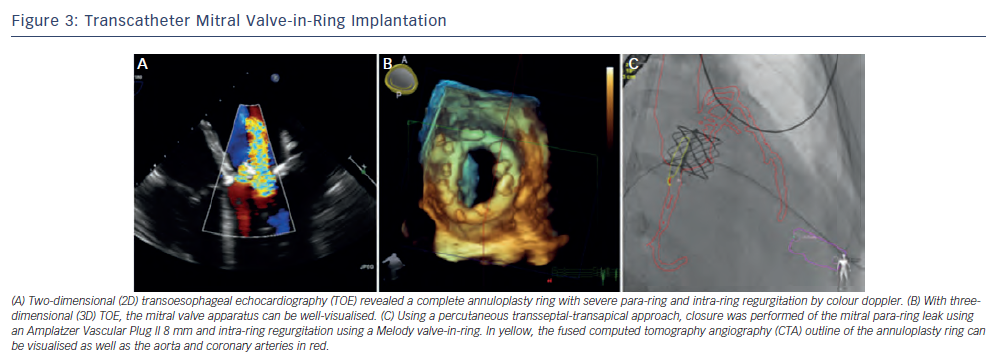
The Global ViV Registry with nearly 120 patients included for Sapien mitral ViV and ViR, reported 30 day and one-year mortality rates of 12 % and 25 %, respectively.17,34 A majority of implantations, over two-thirds, were performed using a surgical transapical approach. Approximately 5 % had device malpositioning related predominately to the difficulties in achieving coaxial deployment through an antegrade transseptal technique. A newer approach of antegrade transseptal utilising a percutaneous transapical rail provides a complete percutaneous approach to mitral ViV and ViR implantation where creation of the arteriovenous rail has allowed for coaxial deployment and improved fine-tuning of valve positioning within the surgical platform35 (see Figures 2 and 3). Radiopaque fluoroscopic markers of the valve can aid in ViV/ViR positioning. The use of computed tomography angiography (CTA) fluoroscopy (HeartNavigator [Philips Healthcare, Best, The Netherlands]) and TOE-fluoroscopy fusion imaging can provide additional landmarks (i.e. sites of transseptal puncture, transapical puncture, mitral bioprosthesis and mitral ring) to perform guided puncture and valve deployment.