The Rationale for Renal Sympathetic Denervation in Human Resistant Hypertension
Activation of the sympathetic nervous system (SNS) is now recognised to occur in all stages of hypertension and correlates to the severity of hypertension.11,12 The renal efferent and afferent SNS neural fibres make their own contribution to the maintenance of the hypertensive phenotype. Renal SNS afferents run along the renal artery adventitia and cluster in the renal pelvis13,14 and activity of these renal SNS fibres regulate whole body sympathetic tone by moderating hypothalamic activity.15 Renal SNS efferents innervate the kidneys from the paravertebral ganglia at T10-L2 and also run alongside the renal afferents in the renal artery adventitia.13 Renal SNS efferent activity mediates renal sodium retention, volume expansion16 and stimulates the neuro-humoral renin-angiotensin-aldosterone axis, further elevating blood pressure (BP) though salt/water retention and vasoconstriction.17
In the first half of the 20th century, surgical thoracolumbar sympathectomy (resulting in renal sympathectomy) was performed to treat malignant hypertension with good results in terms of reducing both BP and mortality.18,19 However, this radical surgical operation was not without significant operative mortality and post-operative morbidity, including postural hypotension, erectile dysfunction and syncope.
Thus the procedure fell out of favour with the advent of antihypertensive medications that were non-invasive, tolerable and proved to reduce BP and mortality.20
Preclinical studies have clearly demonstrated that interruption of renal SNS signalling, by either surgical ligation and re-anastomosis or chemical adventitial stripping of the renal artery, prevents the development of hypertension and, furthermore, attenuates established hypertension in numerous animal models of hypertension.21 Further evidence for benefit of renal sympathectomy in the treatment of hypertension comes from a study of patients treated with bilateral nephrectomy for end-stage chronic kidney disease (CKD) maintained on haemodialysis or post-transplantation, which demonstrates sustained BP reduction.22 While these techniques are not suitable for use in humans, the recent development of minimally invasive, catheter-based solutions10,23 to effect selective RSD has re-ignited interest in this field.
Current Technologies for Renal Sympathetic Denervation
The paradigm for a technology that may have utility in renal SNS modulation for hypertension is that it achieves selective RSD with no collateral damage to adjacent/other structures. The different technologies that are currently being tested in both preclinical and clinical studies vary in their potential benefits and pitfalls and these issues are discussed below.
Radio-frequency Neural Ablation
Radio-frequency (RF) energy, introduced for ablation of neurovascular tissue more than 25 years ago,24 is an alternating electrical current that produces tissue destruction by both direct resistive heating of the tissue in contact with the catheter tip and by thermal conduction to deeper tissue. The RF electrical current is delivered most frequently in the unipolar mode, with completion of the electrical circuit via another electrode placed on the skin. In bipolar mode, two closely opposed electrodes are placed on the catheter electrode tip. On energy delivery to the target surface, the catheter tip heats subjacent (up to 4 mm) tissues from 50 to 70°C.25 A sudden rise in impedance can suggest over-heating and charring of tissue at the tip and many modern RF catheters are designed with auto-feedback mechanisms to prevent excessive temperature elevations. Other factors that influence tissue destruction include duration of energy application, with at least 35 seconds required for uniform temperature elevations in targeted tissue,26 catheter electrode size and tissue apposition and the level of power applied from the RF generator.
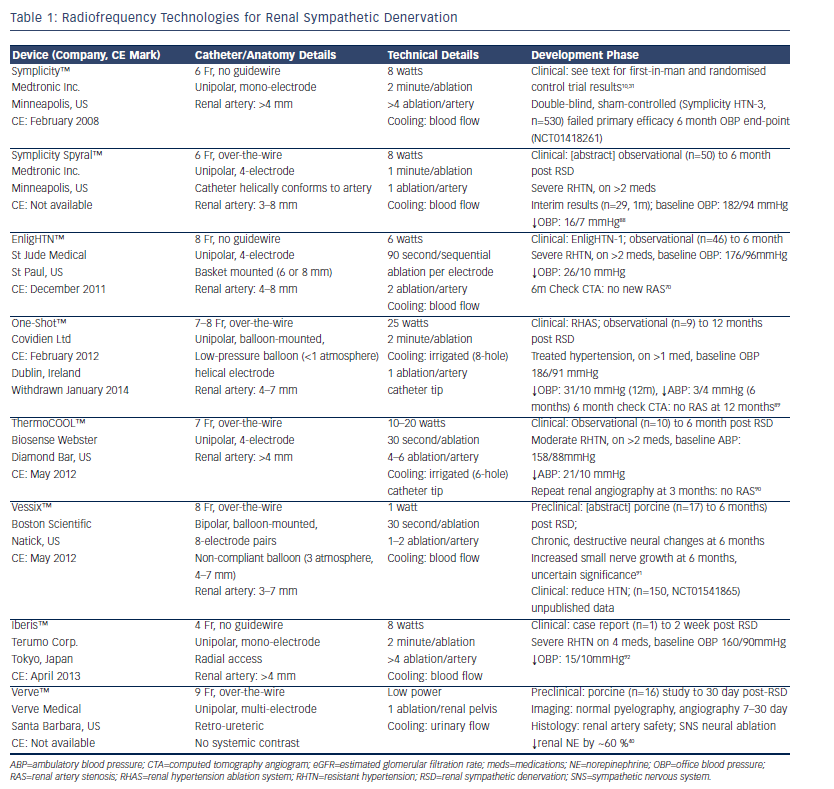
Anatomical considerations are required before progressing to RF RSD.27-29 Prior renal artery duplex scanning or cross-sectional imaging to rule out significant renal atherosclerotic disease is required, and this is confirmed at the time of endovascular RSD by formal angiography before ablation catheter placement. The main trunk diameter should be >4 mm and the length should be >20 mm to allow both effective blood flow for cooling (see below) and sufficient space for multiple ablations. Furthermore, accessory or dual arteries should be of similar dimensions to allow treatment to be given to all arteries concurrently. To date, RF RSD is not recommended in patients with previous renal artery angioplasty or endovascular stents to treat previous atherosclerotic renal artery stenosis (RAS).
First-generation RF RSD systems utilise flexible RF catheters that are advanced into each renal artery in turn under fluoroscopic guidance. Energy delivery causes thermal destruction of SNS neural tissue in the perivascular adventitia and using native renal blood flow to cool the intima, endothelial damage is reduced. Intra-procedural utilisation of vasodilators, such as nitroglycerine (glyceryl trinitrate) and non-dihydropyridine calcium channel blockers, are often used to prevent vasospasm that may accompany energy delivery. The perivascular neural bundle also contains sensory C fibres and thus neural destruction is accompanied by significant pain, necessitating conscious sedation and adequate opiate-based analgesia.
Ardian Inc. (later purchased by Medtronic Inc.) developed the first minimally invasive technology to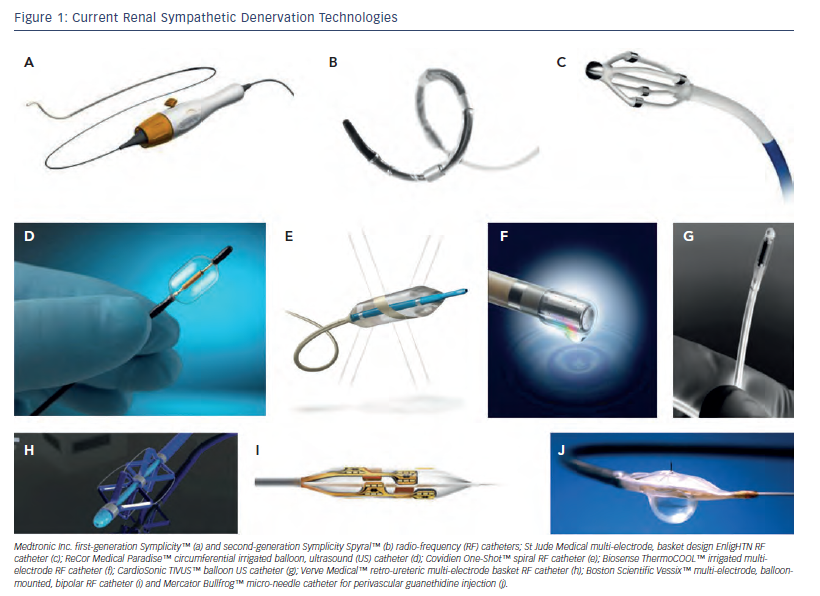 effect selective RSD. The Symplicity™ (Minneapolis, US) catheter consists of a unipolar ablation catheter and a proprietary low-energy RF generator, and is the most widely used and studied device to date. Typically four to seven ablations (5 mm apart; 2 minutes per ablation) are performed sequentially in each artery in a classic helical pattern distally to proximally to prevent potential RAS and cover the full arterial circumference.
effect selective RSD. The Symplicity™ (Minneapolis, US) catheter consists of a unipolar ablation catheter and a proprietary low-energy RF generator, and is the most widely used and studied device to date. Typically four to seven ablations (5 mm apart; 2 minutes per ablation) are performed sequentially in each artery in a classic helical pattern distally to proximally to prevent potential RAS and cover the full arterial circumference.
Symplicity HTN-1 was a non-randomised, first proof-of-concept study using the Symplicity system in severe RHTN (n=45; office BP=177/101 mmHg; mean anti-hypertensive medications=4.7) demonstrating improvement of office BP by 27/17 mmHg at 12-months10 and by 32/14 mmHg at 36 months.30 Symplicity HTN-2 was a randomised trial of RSD (using the Symplicity catheter) plus current treatment versus current treatment only. In patients randomised to RSD (n=49; office BP=178/97 mmHg; mean anti-hypertensive medications=5.2), 6-month office BP-lowering was 32/12 mmHg compared with 1/0 mmHg in controls (n=51).31
Since the publication of these initial studies using the Symplicity catheter, other devices have quickly come to the market and tried to establish their own safety and efficacy profiles (see Figure 1; Tables 1-3), with improved technological iterations on the original Ardian Inc. design. Recently, the first dedicated radial-approach RSD device has gained a CE mark (Iberis™, Terumo Corp, Tokyo, Japan) (see Table 1). This is a unipolar electrode similar to the Symplicity system that is introduced via the trans-radial approach rather than the trans-femoral approach. This trans-radial approach has been shown to be associated with reduced access-site complications in percutaneous coronary interventions (PCI) and is recommended as the default site for access in PCI.32
 Several companies have designed multi-electrode or elongated, spiral electrode catheters, including a second-generation catheter from Medtronic Inc., which can produce simultaneous energy applications at multiple anatomical sites within the renal artery, either mounted externally on a scaffold or inflatable balloon (see Table 1). Not only does this substantially reduce procedure time and contrast load but it may also help achieve complete circumferential nerve ablation, as the catheter does not need to be re-positioned between energy applications. Even more recently, 3D electrical current mapping technology, commonly applied in cardiac electrophysiology (EP) procedures, has been used to further reduce both contrast load and radiation exposure.33
Several companies have designed multi-electrode or elongated, spiral electrode catheters, including a second-generation catheter from Medtronic Inc., which can produce simultaneous energy applications at multiple anatomical sites within the renal artery, either mounted externally on a scaffold or inflatable balloon (see Table 1). Not only does this substantially reduce procedure time and contrast load but it may also help achieve complete circumferential nerve ablation, as the catheter does not need to be re-positioned between energy applications. Even more recently, 3D electrical current mapping technology, commonly applied in cardiac electrophysiology (EP) procedures, has been used to further reduce both contrast load and radiation exposure.33
Further iterations of RF RSD devices include integrated cooling mechanisms to prevent local tissue heating to excessive levels (see Table 1). First-generation systems have utilised concurrent renal artery blood flow to aid cooling of the endothelium to prevent thermal damage. Computational modelling has recently indicated that the intrinsic rate of renal artery blood flow, which cannot be easily manipulated peri-procedurally, is crucial in controlling both the direct, local (i.e. thermal effects to arterial wall) and distant (i.e. thermal effect on blood) effects of RF RSD.34 To counteract these effects, saline-irrigated RF catheters have become a standard design for cardiac EP ablations and have been shown to reduce contact-tissue heating without reducing the destruction of deep target tissue.35 Preliminary preclinical data in swine suggest that irrigated RF RSD ablation using the ThermoCOOL™ system (Biosense Webster Inc. [Diamond Bar, US] [see Table 1]) reduced arterial media and peri-arterial collagen damage but produced similar neural destruction compared with non-irrigated RF RSD procedures in arteries harvested 10 days post-procedure.36
Endothelial damage is a serious concern with non-irrigated RF RSD devices as it has been demonstrated with the use of optical coherence tomography (OCT) imaging that first-generation RF RSD catheters (Symplicity and EnligHTN™ [St Jude Medical, St Paul, US] systems) caused diffuse renal artery vasospasm, local tissue oedema and thrombus,37,38 suggesting the potential need for concurrent, periprocedural anti-platelet therapy.37 This may well be a temporary phenomenon and the clinical significance of these imaging findings is not currently known. Reassuringly, preclinical porcine studies using the Symplicity catheter showed no significant RAS, smooth muscle hyperplasia or thrombosis angiographically or histologically at 6 months post RF RSD.23 Follow-up renal imaging in the Symplicity trials has indicated only one novel RAS as a sequela of RF RSD in 88 patients followed for up to 3 years.30 Furthermore, renal safety has recently been explored in 15 patients with RHTN and moderate to severe CKD stages 3-4) that would have been excluded from the Symplicity HTN-1 and HTN-2 trials. This study revealed preservation of renal function to 12 months after RSD,39 which provides limited but further encouraging data regarding renal safety.
The potential damage caused by RF energy application direct to the renal artery endothelium means that it may not be the optimal technology for endovascular RSD. Other non-RF technologies, described below, are being developed to overcome some of these concerns. Interestingly, the proximity of the renal nerves to the renal pelvis has led to the development of a non-endovascular approach to RF-mediated RSD. Verve Medical (Santa Barbara, US) have developed an eponymous, retro-ureteric delivered RF device that has been tested in preclinical porcine studies, with reduction in renal tissue norepinephrine (NE) levels and no significant vascular or renal parenchymal damage up to 30 days post-procedure.40 This approach prevents patient exclusion for renal arterial anatomical reasons that was common in the Symplicity RSD clinical studies,10,31 but other urological pathologies may prevent usage of this approach in certain patients.