Ultrasound Neural Ablation
Ultrasound (US) energy delivers sound waves >20 Hz. When US is directed against a medium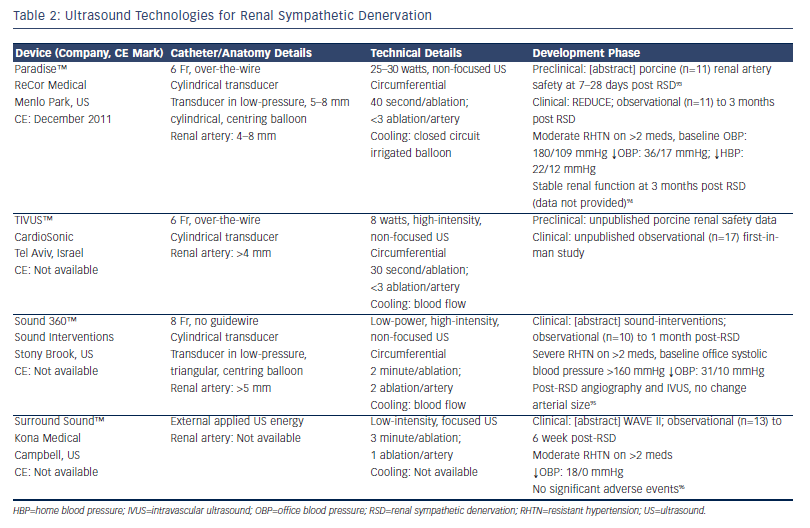 that is able to absorb the energy, it is converted to thermal energy within that medium. It can be delivered without vessel contact, with US waves passing through fluid/interposing tissue to heat target tissue to generate targeted thermal injury. It has been established as an effective therapy for cardiac EP procedures.41 Different approaches have been developed to harness the potential utility of US for RSD with the proposed benefits over RF ablation being controlled and greater depth of denervation and endothelial sparing (see Table 2). The requisite depth for effective denervation is, however, debatable as the majority of human SNS fibres have been shown to lie within 2 mm of the renal arterial lumen14 and deeper denervation techniques may pose harm to adjacent structures including the psoas muscle (posteriorly) and bowel within the peritoneal space (anteriorly).
that is able to absorb the energy, it is converted to thermal energy within that medium. It can be delivered without vessel contact, with US waves passing through fluid/interposing tissue to heat target tissue to generate targeted thermal injury. It has been established as an effective therapy for cardiac EP procedures.41 Different approaches have been developed to harness the potential utility of US for RSD with the proposed benefits over RF ablation being controlled and greater depth of denervation and endothelial sparing (see Table 2). The requisite depth for effective denervation is, however, debatable as the majority of human SNS fibres have been shown to lie within 2 mm of the renal arterial lumen14 and deeper denervation techniques may pose harm to adjacent structures including the psoas muscle (posteriorly) and bowel within the peritoneal space (anteriorly).
While extra-corporeal high-intensity focused US (HIFU) has long been used to ablate deep, solid tissue tumours through thermal injury42 and has recently been tested in preclinical canine studies of RSD,43 the use of extra-corporeal, low-intensity focused US (LIFU) for RSD represents an entirely unique and potentially non-invasive strategy that is particularly attractive (see Table 2). The mechanism of tissue damage by LIFU is not entirely characterised and is thought to be predominantly sono-mechanical (i.e. vibration-induced cellular damage) rather than thermal.44 Although the Surround Sound™ system from Kona Medical (Campbell, US) is currently using a targeting catheter in its early phase development, the stated aim of the company is to develop a fully non-invasive technology that applies LIFU without the requirement for endovascular access. One could imagine such a technology being easily translatable to an ambulant patient setting, which would be the Holy Grail of RHTN therapy.
 Chemical Neural Ablation
Chemical Neural Ablation
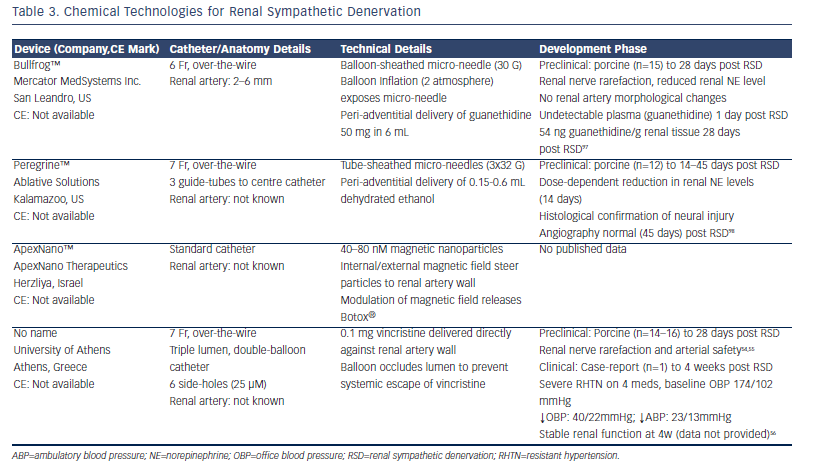
Therapeutic pharmacological neurolysis has been recognised for over a century and several pharmacological agents are being developed for RSD (see Table 3). Targeted drug delivery is an attractive method offering selective neurolysis and obviating endothelial and deeper vascular damage. Alcohol is an effective neurolytic,45 previously used for trigeminal neuralgia,46 and, in fact, more than 20 years ago47 to treat renovascular hypertension through percutaneous injection into the renal artery adventitia. Botulinum toxin type A (commonly known as Botox®) or type B are responsible for the flaccid paralysis associated with Clostridium botulinum poisoning, and have been developed as therapeutic neurolytics to treat muscular dystonias48 and spasticity.49 Similar neurotoxins have been packaged in magnetic nanoparticles that provide a mechanism for targeted drug delivery when combined with an external magnetic field, and have been successfully applied to cardiac SNS ganglionic plexi to treat atrial fibrillation.50 Guanethidine, one of the first anti-hypertensive medications, reduces norepinephrine (NE) levels in pre-synaptic nerve terminals. At higher systemic doses it has been shown to cause selective SNS neurolysis51 through an immune-mediated mechanism.52 The anti-neoplastic vinca alkaloid, vincristine, is well recognised to be neurotoxic, especially to peripheral nerves with systemic application,53 and while this can cause disabling peripheral neuropathies in cancer patients, this medication has been re-tasked for therapeutic usage as an RSD agent, and is the only chemicalbased RSD technology that has produced both preclinical and firstin- man data in peer-reviewed publications.54-56
Cryoablation to Achieve Renal Sympathetic Denervation
Cryotherapy, an effective ablation technology, cools target tissue to 40°C, which results in intra-cellular ice crystal formation and cell death57 and has been used to destroy non-epithelial tissue for over 50 years.58 Cryoablation has become a mainstay for cardiac EP studies, as there is a reduced frequency of vascular complications59 and reduced pain60 compared with standard RF techniques. Standard cardiac EP cryoablation catheters have been used to determine the safety of this approach in preclinical studies that demonstrated effective RSD with no macroscopic evidence of endovascular thrombi, renal parenchymal or vascular damage and endothelial cell preservation by immunohistochemistry.61 In a small pilot study in patients with RHTN deemed non-responders to RF RSD, cryoablation caused appreciable BP reductions in all three patients to 6 months post-procedure (>22/4 mmHg ambulatory BP).62 This approach is being developed by commercial ventures, including CryoMend Inc. and Cryomedix Inc. (both San Diego, US) although there have been no preclinical or clinical data outputs to date. It is too early to speculate on the long-term potential for cryoablation in RSD and on a cautionary note, higher rates of recurrence after successful index ablation are apparent for certain cardiac arrhythmias compared with RF ablation.60
Ionising Radiation Neural Ablation
Radio-ablation is also being developed for endovascular RSD. Although peripheral nerves were initially thought to be relatively radio-resistant, it was established more than 50 years ago that ionising radiation induced neural fibrosis and myelin loss, leading to symptomatic neuropathies in cancer patients treated with radiotherapy.63 Novel, radiation-based therapies that are being developed for RSD include endovascular-radiation brachytherapy (25-50 Gy delivered by Best Vascular Inc., [Springfield, Virginia] Novoste™ Beta-Cath™ catheter), which has demonstrated effective neural destruction and renal artery safety at 25-50 Gy in swine (n=10) up to 2 months post-procedure,64 and stereotactic, non-invasive, robot-assisted, X-radiation radiosurgery (Cyberheart Inc., [Sunnyvale, US] Cyberheart system™), based on the same company's Cyberknife™ system, used to treat solid organ tumours. No preclinical data have been presented to date.