 Use of Bioresorbable Vascular Scaffold in Routine Clinical Practice
Use of Bioresorbable Vascular Scaffold in Routine Clinical Practice
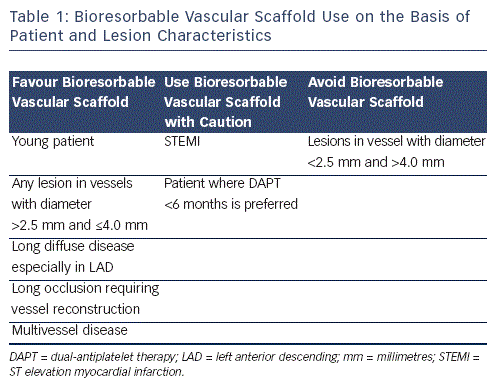
Although most studies to date have evaluated BVS implantation for short, non-calcified lesions in moderately-sized vessels, it is likely that these novel devices, assuming that they fulfil their full potential, will offer most of their benefit in patients with more complex lesions (see Table 1). Thus BVS use should not be restricted to patients with simple (American Heart Association/ American College of Cardiology [AHA/ACC] Type A or B) lesions as such patients would probably have experienced similar benefits with the implantation of conventional metallic DES. This of course does not mean that these patients should not be treated with BVS since the absence of a permanent implant and restoration of vasomotion can still be advantageous as this may reduce the risk of very late ST whilst normal coronary physiology is also returned. Patients with long segments of diffuse disease, long occlusions requiring vessel reconstruction and those requiring multivessel revascularisation can particularly benefit from BVS implantation since stent length returns to zero after resorption (see Figure 1). This is important not only because it permits subsequent percutaneous treatment without the addition of more stent layers whilst also keeping the option of coronary bypass graft surgery open, but also because it can reduce late adverse events such as ST and clinically significant restenosis, the risk of which rises with increasing total stent length. Furthermore, in these patients any 'jailedÔÇÖ side branches following BVS implantation are liberated as the scaffold is resorbed. Thus BVS should also be considered for lesions that involve side  branches, which are too small for dilatation following stent implantation. It is important to mention that when treating patients with long disease to try and limit BVS overlap since currently available devices have much thicker struts (Ôëê150 micrometres [╬╝m]) as compared with conventional DES. Thus in areas of overlap, 300 ╬╝m of BVS surround the vessel, which can impact significantly on lumen area especially in smaller vessels. This can be achieved by first deciding whether to implant proximal to distal or vice versa, something that largely depends on whether greater accuracy is needed for the proximal or distal landing zone. In cases where the proximal landing zone is short it is best to implant first proximally whereas the opposite should be performed with a short distal landing zone. Large overlap can also be avoided by placing the balloon marker of the BVS about to be implanted side-by-side with the platinum marker of the already implanted BVS, since the platinum markers are within the BVS edges.
branches, which are too small for dilatation following stent implantation. It is important to mention that when treating patients with long disease to try and limit BVS overlap since currently available devices have much thicker struts (Ôëê150 micrometres [╬╝m]) as compared with conventional DES. Thus in areas of overlap, 300 ╬╝m of BVS surround the vessel, which can impact significantly on lumen area especially in smaller vessels. This can be achieved by first deciding whether to implant proximal to distal or vice versa, something that largely depends on whether greater accuracy is needed for the proximal or distal landing zone. In cases where the proximal landing zone is short it is best to implant first proximally whereas the opposite should be performed with a short distal landing zone. Large overlap can also be avoided by placing the balloon marker of the BVS about to be implanted side-by-side with the platinum marker of the already implanted BVS, since the platinum markers are within the BVS edges.
Young patients are also attractive candidates for BVS for exactly the same reasons as mentioned above. Patients that require a dedicated two-stent technique for the treatment of a coronary bifurcation may also fare better with a BVS especially when a mini-crush or T-stenting technique is undertaken (see Figure 2). It is important when considering BVS for this indication to ensure that the side branch diameter is 2.5 mm as this is the smallest BVS available, and that the main branch can accommodate 450 µm of struts on the other side of the carina, which will result after a mini-crush technique. Although BVS use in this setting can be slightly challenging, it can be achieved by ensuring adequate predilatation with a balloon of the same diameter as the BVS intended to be implanted. This applies for any lesion to be treated with BVS as it helps to ensure complete BVS expansion. The advantage of using BVS for a dedicated two-stent strategy is that the congestion of struts after a mini-crush technique is undertaken stops being an issue as the scaffold undergoes hydrolysis. It is important to note here that we do not advocate the use of a culotte technique with BVS as these result in relatively long segments of BVS overlap.
Although BVS can be used for most lesions, there are certain occasions that these should be avoided or at least used with caution. However, this does not  include patients with heavily calcified lesions, as currently available tools such as rotational atherectomy, scoring, cutting and ultra-high pressure balloons can ensure that a calcified lesion is well-prepared for BVS implantation. This is a principle that applies also to conventional DES. In our experience, excellent results can be obtained with BVS in such lesions when meticulous lesion preparation as well as post-dilatation have been performed. Occasions where BVS use may not be appropriate includes the treatment of lesions where vessel diameter is <2.5 mm not only because a 2.25 mm BVS is not available but also because the thicker struts of currently available devices may lead to significant lumen reductions. They should also not be used when vessel diameter is >4.0 mm as the largest available BVS is 3.5 mm and the maximum recommended balloon diameter that can be used for this is 4.0 mm (0.5 mm tolerance). In our opinion, they should also be used with caution in STEMI until evidence with regards to BVS use in this context becomes available. Finally, although the use of BVS can theoretically allow shorter periods of dual-antiplatelet therapy (DAPT) due to the inherent biocompatibility of the device, it is not yet known whether this is possible or not. Thus, for patients in whom a DES is required and DAPT cannot be given for more than six months it is perhaps judicious to use a second generation metallic DES for which a shorter DAPT period is possible such as the XIENCE PRIME or XIENCE V (Abbott Vascular, Santa Clara, California, US) or the Endeavor Resolute (Medtronic, Santa Rosa, California, US).
include patients with heavily calcified lesions, as currently available tools such as rotational atherectomy, scoring, cutting and ultra-high pressure balloons can ensure that a calcified lesion is well-prepared for BVS implantation. This is a principle that applies also to conventional DES. In our experience, excellent results can be obtained with BVS in such lesions when meticulous lesion preparation as well as post-dilatation have been performed. Occasions where BVS use may not be appropriate includes the treatment of lesions where vessel diameter is <2.5 mm not only because a 2.25 mm BVS is not available but also because the thicker struts of currently available devices may lead to significant lumen reductions. They should also not be used when vessel diameter is >4.0 mm as the largest available BVS is 3.5 mm and the maximum recommended balloon diameter that can be used for this is 4.0 mm (0.5 mm tolerance). In our opinion, they should also be used with caution in STEMI until evidence with regards to BVS use in this context becomes available. Finally, although the use of BVS can theoretically allow shorter periods of dual-antiplatelet therapy (DAPT) due to the inherent biocompatibility of the device, it is not yet known whether this is possible or not. Thus, for patients in whom a DES is required and DAPT cannot be given for more than six months it is perhaps judicious to use a second generation metallic DES for which a shorter DAPT period is possible such as the XIENCE PRIME or XIENCE V (Abbott Vascular, Santa Clara, California, US) or the Endeavor Resolute (Medtronic, Santa Rosa, California, US).