Current Experience with Bioresorbable Vascular Scaffold in Bifurcation Lesions
BVS has been intensely investigated in two cohort studies (ABSORB Cohort A and B) enrolling patients with relatively simple coronary lesion complexity.16-18 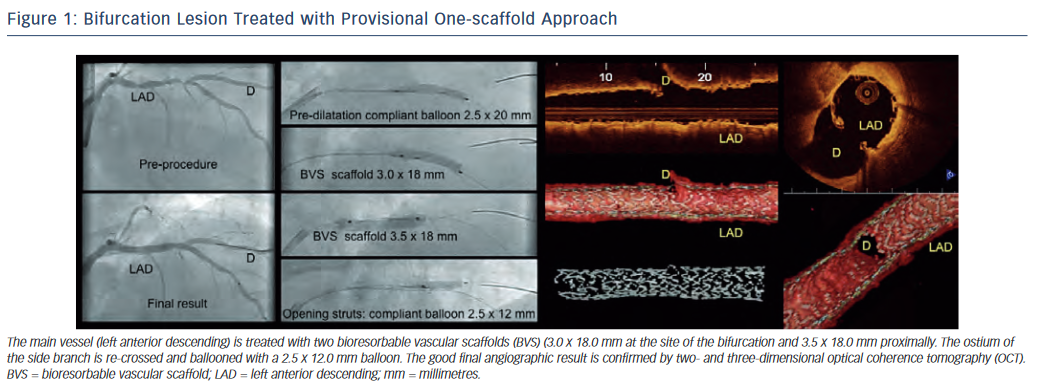
On the 1st September 2012, the BVS was commercially launched in the Netherlands. The Erasmus Medical Center immediately started with the BVS Expand Registry to prospectively follow up procedural and clinical data in a wider pallet of coronary lesions including bifurcations, chronic total occlusions and calcified lesions.16,19
Analogous to the mainstream bifurcation stenting paradigm, the provisional one-scaffold technique is considered the approach of first choice. One scaffold is implanted in the main vessel followed, if necessary, by balloon inflation of the side branch with or without final kissing balloon (see Figure 1). Okamura et al. illustrated balloon dilatation through the BVS cells and the appearance of the fenestrated scaffold20 in an in vitro model. Our group demonstrated the feasibility of BVS re-crossing into the side branch with a coronary guidewire and subsequent ballooning of the side branch ostium through the scaffold cells in vivo.21,22
Over a 10-month timeframe we treated approximately 60 patients with bifurcation lesions. We used a two-scaffold technique in three cases (two with a modified T-technique and one with the culotte technique). In one-third of cases the scaffold struts into the side branch was rewired and dilated. Procedural and acute clinical success was achieved in all cases and thus compares favourably to that with standard metallic stents. Specific adjustments to established bifurcation techniques were avoidance of simultaneous kissing balloon inflations and aggressive proximal optimisation techniques in order to avoid proximal scaffold damage. Furthermore we currently advocate a low threshold to confirm the final scaffold result by invasive imaging.