Clinical Studies Involving Platelet Reactivity Testing
Testing platelet reactivity following the administration of antiplatelet agents aims to determine how the drugs affect their biological targets, which is likened to the  risk for secondary ischaemic/bleeding events. It is important to evaluate the levels of platelet reactivity that may correlate with thrombotic events and to create a clinically meaningful cut-off value of platelet reactivity. According to the VerifyNow package insert, the highest combination of sensitivity and specificity for identifying a measureable effect of a P2Y12 inhibitor is a PRU of 208.36 To date, cut-off values have mainly been investigated in patients undergoing PCI. However, platelet reactivity testing has been employed in numerous clinical indications (see Table 1).
risk for secondary ischaemic/bleeding events. It is important to evaluate the levels of platelet reactivity that may correlate with thrombotic events and to create a clinically meaningful cut-off value of platelet reactivity. According to the VerifyNow package insert, the highest combination of sensitivity and specificity for identifying a measureable effect of a P2Y12 inhibitor is a PRU of 208.36 To date, cut-off values have mainly been investigated in patients undergoing PCI. However, platelet reactivity testing has been employed in numerous clinical indications (see Table 1).
Peripheral Angioplasty Procedures
Clopidogrel resistance, defined as PRU, significantly affected clinical outcomes after peripheral angioplasty procedures.37,38
Coronary Artery Bypass Graft Surgery
A strategy based on pre-operative platelet reactivity testing to determine the timing of CABG surgery in clopidogrel-treated patients was associated with the same amount of bleeding observed in clopidogrel-naive patients and 50 % shorter waiting time than recommended in the current guidelines.39 These results led to an upgrade in the guidelines, to base timing of surgery on platelet function monitoring rather than the arbitrary use of a specified period of delay.40
Neurovascular Applications
Clopidogrel resistance, defined using a PRU cut-off value of 235, was associated with increased periprocedural thromboembolic complications following neurovascular stenting.41 Flow diversion with the Pipeline™ Embolization Device (PED) is an important treatment option for cerebral aneurysm, but it is associated with the risk of thromboembolic complications. A study of pre-procedure platelet reactivity testing using VerifyNow found that a PRU value of <60 or >240 was the strongest independent predictor of all major peri-operative thromboembolic and haemorrhagic complications after PED procedures.23 In defining optimal cut-off values, it is also important to distinguish between a cardiovascular and neurovascular patient - those with a history of stroke have a greater risk of haemorrhage on more potent antiplatelet therapy and may benefit from platelet reactivity within a more narrow window.42
Percutaneous Coronary Intervention
 The level of platelet function inhibition as measured by a point-of-care assay is an independent predictor for the risk of major adverse cardiac events after PCI.43 A recent systematic review and meta-analysis concluded that initiating intensified antiplatelet therapy on the basis of platelet reactivity testing in patients after PCI is associated with a significant reduction in cardiovascular mortality, ST and MI (p<0.01 for all).44
The level of platelet function inhibition as measured by a point-of-care assay is an independent predictor for the risk of major adverse cardiac events after PCI.43 A recent systematic review and meta-analysis concluded that initiating intensified antiplatelet therapy on the basis of platelet reactivity testing in patients after PCI is associated with a significant reduction in cardiovascular mortality, ST and MI (p<0.01 for all).44
A study of patients with ACS assessed the cost-effectiveness of universal clopidogrel, ticagrelor or prasugrel (given to all patients) or PRA-driven ticagrelor or prasugrel (given to patients with a PRU of >230 on the VerifyNow assay; the remainder received clopidogrel). PRA-driven ticagrelor and prasugrel were found to be cost-effective over five years compared with universal clopidogrel (incremental cost-effectiveness ratio US$40,100 and US$49,143/quality-adjusted life-year, respectively); however, universal ticagrelor and prasugrel were not cost-effective (incremental cost-effectiveness ratio US$61,651 and US$96,261/quality-adjusted life-year, respectively).45 Thus, platelet reactivity testing has the potential to decrease the healthcare costs associated with ACS.
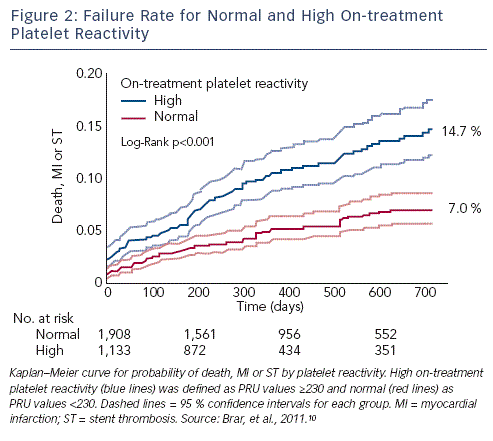
Defining optimal cut-offs has proved challenging in clinical studies of antiplatelet therapy following PCI. A study assessed post-clopidogrel HTPR using VerifyNow following PCI with drug-eluting stent implantation and established a cut-off of 235 PRU.46 Stratifying patients according to HTPR based on this threshold proved strongly predictive of cardiovascular events. A recent meta-analysis of clinical outcomes after PCI confirmed this finding and found that every 10-U increase in PRU was associated ith a significantly higher rate of the primary endpoint (hazard ratio [HR] 1.04; 95 % confidence interval (CI) 1.03-1.06; p<0.0001). A PRU cut-off of 230 was the strongest predictor of death, MI or ST (p<0.001). A PRU value 230 was associated with a higher rate of a composite primary endpoint of death, MI or ST (HR 2.10; 95 % CI 1.62-2.73; p<0.0001), as well as the individual endpoints of death (HR 1.66; 95 % CI 1.04-2.68; p<0.04), MI (HR 2.04; 95 % CI 1.51-2.76; p<0.001) and ST (HR 3.11; 95 % CI 1.50-6.46; p<0.002) (see Figure 2).10
As mentioned previously, according to the VerifyNow package insert, the highest combination of sensitivity and specificity for identifying a measureable effect of a P2Y12 inhibitor is a PRU of 208.36 However, among clinical studies, a consensus has not yet been reached on optimal cut-off values. One small study of patients with stable angina suggested that a cut-off of 15 % inhibition or >213 PRU may be optimal for the identification of patients with HTPR.47 Another study of patients with stable coronary artery disease (CAD) found that cut-off levels of 256 PRU and 26.5 % inhibition identified carriers of reduced-function cytochrome P450 2C19 (CYP2C19) allele on antiplatelet therapy, which is associated with a higher rate of clinical risk.48,49 As the prevalence of the CYP2C19 variant is higher in Asian population compared with the prevalence in Westerners, PRU 275 has predicted clinical events more accurately in this population.50,51 For low-risk patients, lower PRU values (180-210) have been used.18,52 However, the recommended cut-off of 208 was found to be significantly associated with the risk of ST at 30 days (HR 3.9, p=0.005) in the Assessment of Dual AntiPlatelet Therapy With Drug Eluting Stents (ADAPT-DES) registry study (n=8,583), which included a higher risk population (~50 % with ACS).26
A recent study found that HTPR decreases from baseline to one month, a trend that is partly influenced by genotype. The study found that HTPR at one month was the strongest predictor of adverse outcomes.53
While platelet activity appears to correlate with the risk of cardiovascular events and ST following PCI, studies investigating the use of platelet reactivity testing to guide treatment have had mixed results.18,44,54,55 The Gauging Responsiveness With A VerifyNow Assay-Impact On Thrombosis And Safety (GRAVITAS) trial investigated a strategy based on high-dose clopidogrel in patients with HTPR (defined as 230 PRU).25 However, this strategy of a fixed higher dose, regardless of the achieved level of platelet inhibition, did not reduce cardiovascular death, MI and ST after PCI compared with standard-dose clopidogrel.25 However, a subsequent analysis of GRAVITAS data found that achievement of <208 PRU was significantly and independently associated with a markedly lower risk of cardiovascular events at 60 days (adjusted HR 0.23; 95 % CI, 0.05-0.98; P=0.047).56 The lower cut-off may be due to the fact that this trial enrolled a relatively low-risk population. Consequently the trial showed low overall event rates. Furthermore, patients were randomised to single or double-dose clopidogrel rather than targeting to a specific inhibition level. Other studies have suggested that a triple dose may be needed in patients exhibiting variants in the CYP2C19 gene.57
The Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel (TRIGGER-PCI) trial, a randomised trial of prasugrel versus clopidogrel in patients with HTPR after PCI, was terminated early after a preliminary, blinded analysis indicated that the trial would not meet its primary endpoints. The rate of adverse ischaemic events was very low in the sample cohort, making it difficult to achieve statistical significance.54 However, the study enrolment was biased towards very low risk populations - approximately 30 % of the study population did not go on to randomisation after it was established that they had HTPR on clopidogrel.
The Assessment by a double Randomization of a Conventional antiplatelet strategy versus a monitoring-guided strategy for drug-eluting stent implantation and, of Treatment Interruption versus Continuation one year after stenting (ARCTIC) trial randomised patients scheduled for coronary stenting to platelet function monitoring with the VerifyNow and adjusting antiplatelet therapy accordingly versus no monitoring and conventional clopidogrel therapy. HTPR was defined as 230 PRU or a percent inhibition <15 %. In the monitoring arm, serial platelet function tests (before stent implantation and during the maintenance phase) and treatment adjustments using a predefined treatment algorithm were performed. In addition to treatment intensification due to high on-treatment platelet reactivity, patients could be switched back from prasugrel to clopidogrel after PCI if low on-treatment platelet reactivity was measured. Despite halving the rate of high platelet reactivity to adenosine diphosphate, the primary endpoint of death, MI, ST, stroke or urgent revascularisation was similar after one year with the two strategies (HR 1.13, 95 % CI 0.98-1.29; p=0.10).58 The risk level of the population, the cut-off value used to define HTPR, the heterogeneity in treatment adjustment (or lack of adjustment) in deemed non-responders, the rare use of prasugrel, which became available late after study initiation, and the lack of impact of treatment adjustment on other major determinants of platelet reactivity (such as treatment compliance) may account for the lack of benefit. However, ARCTIC is the only study that has evaluated by randomisation to the use of a platelet function test. It was appropriately powered to test the hypothesis of treatment monitoring. One-third of the randomised patients had an ACS, and one-year mortality was 2 %. Eight of 10 patients deemed non-responders were reloaded with clopidogrel and were given a glycoprotein IIb/IIIa inhibitor; 22 % of patients with high on-treatment platelet reactivity were on prasugrel at the end of the study. The rate of poor response was halved after treatment adjustment for both P2Y12 inhibition and aspirin pathway, further highlighting an aggressive approach.