Perspectives
Personalised therapeutic strategies based on platelet reactivity testing have yet to improve clinical outcomes in low-risk groups, perhaps due to the already low event rates observed in this patient population. However, it has been hypothesized that the approach may be more beneficial in high-risk patient populations.8,54 In addition  to responsiveness to antiplatelet medication, there are numerous risk factors for future cardiovascular (CV) events following PCI; these are summarised in Table 2. Risk factors for non-response to antiplatelet therapy include advanced age,59 type 2 diabetes,60 particularly if treated with insulin,61 overweight patients,62 genetic factors49,63-66 and concomitant medications, particularly proton pump inhibitors.67,68
to responsiveness to antiplatelet medication, there are numerous risk factors for future cardiovascular (CV) events following PCI; these are summarised in Table 2. Risk factors for non-response to antiplatelet therapy include advanced age,59 type 2 diabetes,60 particularly if treated with insulin,61 overweight patients,62 genetic factors49,63-66 and concomitant medications, particularly proton pump inhibitors.67,68
The Concept of a Therapeutic Window for Antiplatelet Therapy
The concept of a therapeutic window of P2Y12 reactivity defining the upper threshold as HPTR associated with ischaemic events and lower threshold based on bleeding risk has been suggested.28 A tudy using the VerifyNow assay established a therapeutic window of 86-238 PRU at a one month time-point that minimised both ischaemic and bleeding events (see Figure 3). At the time of PCI, the suggested cut-offs were 95 PRU for hyper- and 214 for hypo-response.53
There is a need to target high-risk patients groups to optimise the clinical utility of platelet reactivity testing. Recent American and European guidelines have included Class IIb recommendations for platelet reactivity testing in high-risk patients if the results may impact on patient management.69,70 Adjusting medication based on the results of platelet reactivity testing is already being carried out in some outpatient ST clinics and hundreds of US hospitals.71 It must be stressed that this approach has not been conclusively validated, though it is logical based on the desire to observe evidence of treatment success in patients treated with antiplatelet agents.
The ongoing Assessment of a Normal versus Tailored Dose of Prasugrel after Stenting in Patients Aged >75 Years to Reduce the Composite of Bleeding, Stent Thrombosis and Ischemic Complications (ANTARCTIC) study (ClinicalTrials.gov number: NCT01538446) will assess the value of platelet function testing in older ACS patients, a complex population whereby major bleeds are as frequent as ischaemic events (see Figure 4). An ongoing phase of the ARCTIC trial, ARCTIC-2, will aim to determine the most effective duration of treatment.
Switching Strategies
Prasugrel has been associated with lower platelet reactivity than clopidogrel,72 providing evidence to support the rationale for switching therapies. The PLATelet inhibition and patient Outcomes (PLATO) study found that ticagrelor improved outcomes but did not increase the risk of major bleeding compared with clopidogrel 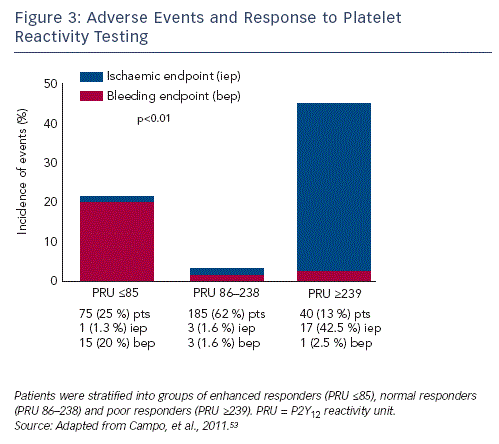 for patients with ACS for whom an early invasive strategy was planned.17 The platelet substudy of the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial found that among patients with ACS without ST-segment elevation who were not treated with revascularisation, prasugrel use was associated with lower platelet reactivity compared with clopidogrel, irrespective of age, weight and dose. However, no significant differences between prasugrel versus clopidogrel in the occurrence of the primary efficacy endpoint, a composite of cardiovascular death, MI or stroke, were found. There was a univariate association between platelet reactivity and ischaemic outcomes that lost significance in an extensive multivariate analysis after adjustment of several factors, including variables associated with on-treatment platelet reactivity.73 Switching strategies have proved that switching from clopidogrel to prasugrel or ticagrelor results in decreased platelet function and is well-tolerated without any major safety events.74 The Randomised, Double-Blind, Outpatient, Crossover Study of the Anti-platelet Effects of Ticagrelor Compared With
for patients with ACS for whom an early invasive strategy was planned.17 The platelet substudy of the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial found that among patients with ACS without ST-segment elevation who were not treated with revascularisation, prasugrel use was associated with lower platelet reactivity compared with clopidogrel, irrespective of age, weight and dose. However, no significant differences between prasugrel versus clopidogrel in the occurrence of the primary efficacy endpoint, a composite of cardiovascular death, MI or stroke, were found. There was a univariate association between platelet reactivity and ischaemic outcomes that lost significance in an extensive multivariate analysis after adjustment of several factors, including variables associated with on-treatment platelet reactivity.73 Switching strategies have proved that switching from clopidogrel to prasugrel or ticagrelor results in decreased platelet function and is well-tolerated without any major safety events.74 The Randomised, Double-Blind, Outpatient, Crossover Study of the Anti-platelet Effects of Ticagrelor Compared With 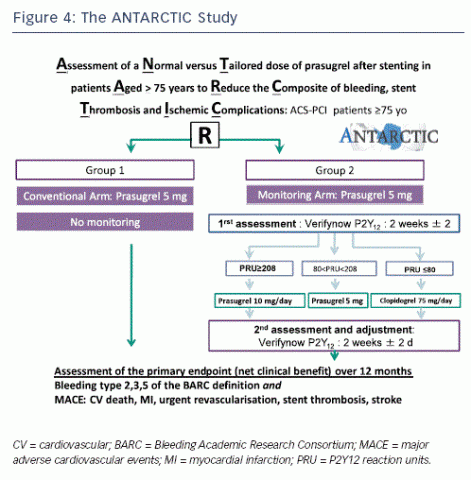 Clopidogrel in Patients With Stable Coronary Artery Disease Previously Identified as Clopidogrel Non-responders or Responders (RESPOND) study found that treatment of clopidogrel non-responders with ticagrelor in a >10 %, >30 % and >50 %, decrease in platelet aggregation from baseline in 100 %, 75 % and 13 % of patients, respectively, and its antiplatelet activity was the same in responders and non-responders. Almost all clopidogrel non-responders and responders treated with ticagrelor had platelet reactivity below the cut-points associated with ischaemic risk.75 The ADAPT-DES study demonstrated that patients with a detectable drug effect on clopidogrel (PRU <208) represent a lower risk cohort than do those with no effect, and as a result, the ischaemic benefit with the newer, more expensive P2Y12 inhibitors will be smaller than that in patients without a detectable drug effect, but will potentially expose them to a greater bleeding risk.26,76
Clopidogrel in Patients With Stable Coronary Artery Disease Previously Identified as Clopidogrel Non-responders or Responders (RESPOND) study found that treatment of clopidogrel non-responders with ticagrelor in a >10 %, >30 % and >50 %, decrease in platelet aggregation from baseline in 100 %, 75 % and 13 % of patients, respectively, and its antiplatelet activity was the same in responders and non-responders. Almost all clopidogrel non-responders and responders treated with ticagrelor had platelet reactivity below the cut-points associated with ischaemic risk.75 The ADAPT-DES study demonstrated that patients with a detectable drug effect on clopidogrel (PRU <208) represent a lower risk cohort than do those with no effect, and as a result, the ischaemic benefit with the newer, more expensive P2Y12 inhibitors will be smaller than that in patients without a detectable drug effect, but will potentially expose them to a greater bleeding risk.26,76
Combining PLATelet Reactivity Testing with Point-ofcare Genetic Testing
The Spartan RX CYP2C19 assay is a point-of-care genetic test that identifies carriers of the CYP2C19 mutation. A prospective, randomised proof-of-concept trial found that the test was specific and sensitive for measuring the genotype, compared to sequencing, and could be performed at the bedside.77 Several larger clinical trials are currently investigating this technique.78-80 Combining platelet reactivity testing with point-of-care genetic testing may provide a personalised approach to guiding treatment with antiplatelet therapies.