The Symplicity™ Renal Denervation Clinical Trial Programme
Early clinical trial data showed that the use of the Symplicity RDN system was associated not only with reductions in systolic BP (SBP) and diastolic BP (DBP) but also with markers of hypersympathetic activity, such as reductions in muscle sympathetic activity and reductions in cardiac baroreex sensitivity,11 as well as a reduction in renal noradrenaline spillover.17
The rst human clinical trial, Renal Denervation in Patients With Uncontrolled Hypertension (Symplicity HTN-1) (n=153), was an aggregate of multiple studies of  patients with resistant hypertension (ofce SBP 160 mmHg with at least three or more antihypertensive medications, including a diuretic) and normal renal function (GFR >45 millilitres per minute [ml/min]).12,17 At six months, 92 % of patients had an ofce BP reduction of 10 mmHg, with reductions in SBP and DBP of 25/11 mmHg, respectively (p<0.0001). Safety data were excellent - 97 % of patients had no complications. The four acute procedural complications included three pseudoaneurysms of the common femoral artery and one renal artery dissection, all managed without further sequelae. Three-year follow-up data showed no treatment-related vascular complications; no hypotensive events that required hospitalisation; no orthostatic hypotension; no electrolyte disturbances; and no signicant changes in mean electrolytes or estimated GFR (eGFR).18 Of the short-term follow-up renal imaging performed, no evidence of renal artery stenosis or abnormalities was noted in treated arteries.
patients with resistant hypertension (ofce SBP 160 mmHg with at least three or more antihypertensive medications, including a diuretic) and normal renal function (GFR >45 millilitres per minute [ml/min]).12,17 At six months, 92 % of patients had an ofce BP reduction of 10 mmHg, with reductions in SBP and DBP of 25/11 mmHg, respectively (p<0.0001). Safety data were excellent - 97 % of patients had no complications. The four acute procedural complications included three pseudoaneurysms of the common femoral artery and one renal artery dissection, all managed without further sequelae. Three-year follow-up data showed no treatment-related vascular complications; no hypotensive events that required hospitalisation; no orthostatic hypotension; no electrolyte disturbances; and no signicant changes in mean electrolytes or estimated GFR (eGFR).18 Of the short-term follow-up renal imaging performed, no evidence of renal artery stenosis or abnormalities was noted in treated arteries.
The successful results of the Symplicity HTN-1 trial were expanded by the multicentre, prospective, randomised Renal Denervation in Patients With Uncontrolled Hypertension (Symplicity HTN-2) trial (n=106), in which patients with resistant hypertension and ofce SBP 160 mmHg (150 mmHg for patients with type 2 diabetes) were randomised to RDN immediately or after six months, without any change in the previous antihypertensive medication regimen. At six months, RDN was associated with BP reductions of 32/12 mmHg (p<0.0001),13 showing superiority over medication management alone, and similar results were reported at two years.19 Recently presented 30-month follow-up data showed 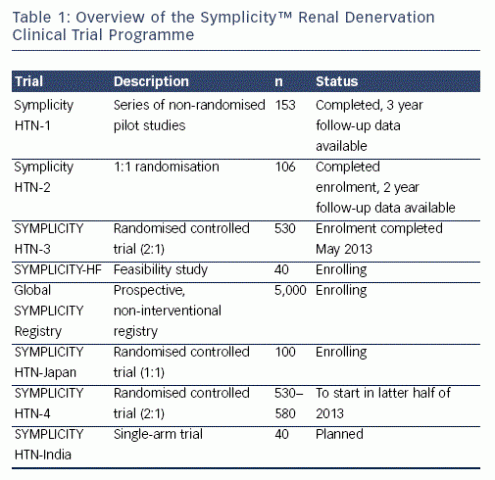 durable BP reductions of 35/13 mmHg (p<0.01) in subjects available at data lock. At six months after randomisation, 46 of the 51 patients available for follow-up crossed over to RDN, 35 of which still met initial eligibility criteria. In the crossover group, a signicant reduction in both SBP and DBP was of almost equal magnitude to that of the initial treatment cohort (see Figure 1).20
durable BP reductions of 35/13 mmHg (p<0.01) in subjects available at data lock. At six months after randomisation, 46 of the 51 patients available for follow-up crossed over to RDN, 35 of which still met initial eligibility criteria. In the crossover group, a signicant reduction in both SBP and DBP was of almost equal magnitude to that of the initial treatment cohort (see Figure 1).20
The Renal Denervation in Patients With Uncontrolled Hypertension (SYMPLICITY HTN-3) trial (n=530) is a multicentre, prospective randomised controlled study, which is blinded and includes a mask blinded (sham) procedure in the study design.21 It will address ambulatory blood pressure monitoring (ABPM) as both an entry criteria and a powered secondary endpoint. In addition, a feasibility study in heart failure (Renal Denervation in Patients With Chronic Heart Failure & Renal Impairment Clinical Trial [SYMPLICITY-HF], n=40), and a hypertension clinical trial in Japan (Renal Denervation by MDT-2211 System in Patients With Uncontrolled Hypertension [HTN-J], n=100) are underway. Further trials in India (Single-arm Study of Symplicity™ Renal Denervation System in Patients With Uncontrolled Hypertension in India) and the US (Renal Denervation in Patients With Uncontrolled Hypertension [SYMPLICITY HTN-4]) are planned (see Table 1). All of these trials, together with the Global Prospective Registry for Sympathetic Renal Denervation in Selected Indications Through 3-5 Years Registry (Global SYMPLICITY Registry) discussed below, will include over 320 sites and nearly 6,000 patients.
In summary, clinical trials to date investigating the safety and efcacy of RDN with the Symplicity RDN system for patients with resistant hypertension have demonstrated signicant and durable reductions in BP; procedural, intermediate and long-term safety as well as preservation of electrolyte and human homeostasis. Ongoing evaluation should conrm the effectiveness of RDN in selected and broader patient populations.