 The Global SYMPLICITY Registry
The Global SYMPLICITY Registry
In addition to the Symplicity clinical trials, the Global SYMPLICITY Registry, which will include 5,000 patients in more than 200 sites worldwide, is being conducted.22,23 Inclusion criteria are patients 18 years and older that are eligible for RDN and sign a patient consent form. The registry will also include patients with conditions characterised by an increase in sympathetic activity, including heart failure, chronic kidney disease, sleep apnoea and atrial brillation. The aims of this registry are to document the long-term safety and effectiveness of RDN; in everyday clinical practice; to monitor BP response in different nationalities and races; to identify patients who are likely to respond best to RDN and to monitor pleiotropic treatment effects, such as changes in glucose metabolism, renal and cardiac function. Secondary objectives include duration of BP lowering after treatment.23
The Global SYMPLICITY Registry is intended as an umbrella under which national registries, including the German Renal Denervation (GREAT) registry in Germany (n=1,000), the Korea Registry (n=102) and the South Africa Registry (n=400), will contribute data. The recommended follow-up schedule is three months, six months, one year, and each year up to ve years after treatment.23 Baseline and follow-up assessments will include patient demographics; physical measurements; ofce and 24-hour ambulatory BP, medication logs; quality of life; and heart rate. Vascular safety in the renal artery will be assessed and right ventricular imaging will also be conducted for those patients who receive cardiac imaging as per their standard of care, since it has been shown that RDN reduces heart rate.24
Patient selection is crucial to the success of RDN therefore subgroup analysis from the registry will be performed to determine whether any patient group especially benets from the procedure. This will include renal function (eGFR <60 versus >60 ml/min/1.73 square metres [m2]). The registry will also compare dippers (patients with lower BP at night) to non-dippers (characterised by an increased sympathetic activity and an indication of higher cardiovascular risk). Analyses will also focus on subgroups with left ventricular hypertrophy (LVH), an indicator of end-organ damage in arterial hypertension. The presence of LVH is associated with an increased rate of cardiovascular events and death independent of BP.25 Subgroup analysis will also include patients with type 2 diabetes, impaired glucose tolerance, hyperinsulinemia, concomitant use of oral sympatholytic drugs, and consider age, heart rate and BP above and below median.
Changes in medication will also be recorded. It is recommended that baseline medication is maintained in order to accurately assess the net effect of RDN on BP, although in practice patients tend to manage their medications themselves. Poor compliance to therapy is a well-known problem in resistant hypertension; a recent study involving toxicological urine analysis found that drug adherence in resistant hypertension was only 47 %.26 This leads to difculties in terms of interpreting BP measurements and in obtaining an accurate diagnosis of resistant hypertension.
Safety endpoints will include vascular complications; renal artery perforation or dissections; renal artery re-interventions; new renal artery stenosis; hypertensive crisis; contrast nephropathy (acute eGFR drop of >25 % or new renal failure); new need for dialysis; and signicant embolic event resulting in end-organ damage. Stroke, acute MI, end-stage renal disease, atrial brillation and mortality will also be investigated. As of January 2013, data were available for 617 patients, the majority (60%) of which had been treated according to the European Society of Cardiology (ESC) consensus guideline paper on RDN.27 Preliminary six-month data demonstrated an excellent procedural and clinical safety prole, including signicant reductions in both ofce and ambulatory BP compared to baseline.23 In summary, the enrolment and analyses of the Global SYMPLICITY Registry continue to meet the goals of establishing the procedural safety and efcacy of RDN.
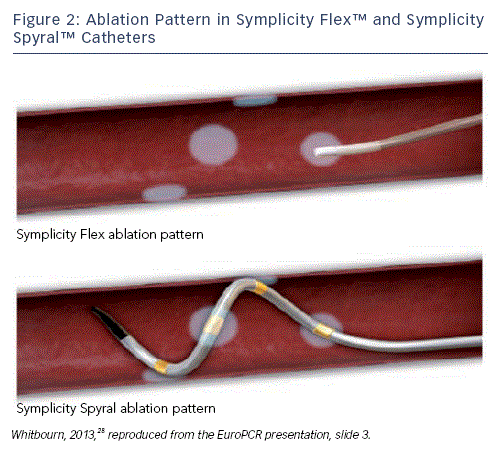
The Symplicity Spyral™ Multi-electrode Renal Denervation Catheter
 Renal denervation using the Symplicity Flex catheter has demonstrated efcacy and safety both in clinical trial and real-world settings; however, it would be desirable to minimise the amount of treatment time required during the procedure. The Symplicity Spyral multi-electrode renal denervation catheter was designed with the goal of reducing procedure time while maintaining similar clinical outcomes and reassurance of success as compared to the original proven Symplicity Flex catheter. The Symplicity Spyral catheter has a helical-shape, and the electrode array consists of four independently selectable RF electrodes radially spaced by approximately 90 degrees to each other. The electrodes deliver energy simultaneously, decreasing the time for the ablation cycle to one minute per artery (see Figure 2) and the commercial catheter can be used for arteries with diameters between 3 and 8 millimetres (mm) ('one-size-ts-all').
Renal denervation using the Symplicity Flex catheter has demonstrated efcacy and safety both in clinical trial and real-world settings; however, it would be desirable to minimise the amount of treatment time required during the procedure. The Symplicity Spyral multi-electrode renal denervation catheter was designed with the goal of reducing procedure time while maintaining similar clinical outcomes and reassurance of success as compared to the original proven Symplicity Flex catheter. The Symplicity Spyral catheter has a helical-shape, and the electrode array consists of four independently selectable RF electrodes radially spaced by approximately 90 degrees to each other. The electrodes deliver energy simultaneously, decreasing the time for the ablation cycle to one minute per artery (see Figure 2) and the commercial catheter can be used for arteries with diameters between 3 and 8 millimetres (mm) ('one-size-ts-all').
Preclinical data using a porcine model showed that the ablation pattern achieved using the Symplicity multi-electrode catheter was consistent with the ablation pattern obtained with the Symplicity single-electrode catheter. At 28 days post-intervention, no difference in norepinephrine levels, a measure of renal sympathetic activity, was seen between the multi-electrode and single-electrode catheter; in both cases there was a signicant reduction compared with the control kidneys. Histological evaluation also revealed no sign of injury to the renal artery.28 A feasibility study is underway to assess the efcacy of the Symplicity Spyral catheter in the acute setting. The feasibility study is expected to include up to 50 patients in total. The inclusion criteria are similar to those used in the Symplicity clinical trials. Initial results in 29 patients were presented at the 2013 EuroPCR meeting in Paris, France. The mean BP at baseline was 182/94 mmHg, and participants were taking 4.7 medications. The mean procedure time for the Symplicity Spyral catheter (calculated as guide catheter removal - catheter insertion) was 21.2 minutes. Nearly all of the patients had an RF treatment time of one minute per artery; two patients received more than one treatment in a single artery. At one month, patients experienced an average ofce BP reduction of 16/7 mmHg from baseline (p<0.001), which was consistent to that achieved in the Symplicity-HTN trials. A reduction in heart rate of 4.3 beats per minute (p<0.047) and a decrease in pulse pressure of 8.8 mmHg (p=0.004) were also seen. The procedure had 96.6 % procedure success (dened as successful delivery of any RF in the absence of an in-hospital major adverse effect). One femoral pseudoaneurysm occurred in hospital at the access site requiring surgical intervention and one occurred at day three post-treatment requiring compression.28
In summary, in data generated to date, the Symplicity Spyral catheter had a safety prole consistent with the safety results demonstrated by the Symplicity Flex catheter; had demonstrated preclinical and clinical efcacy data consistent with the Symplicity Flex catheter; and confers the advantage of shorter treatment duration.
Effectiveness of Renal Denervation in Mild to Moderate Resistant Hypertension
The safety and efcacy of RDN for BP reduction in patients with severe resistant hypertension has been established. However, such patients represent only a small portion of the hypertensive community. Current studies are investigating the possibility of expanding the therapeutic indications for RDN, including the larger 'mild to moderate' resistant hypertension population. An ongoing observational non-randomised trial29 (n=54) included patients with ofce BP above 140/90 mmHg and below 160/100 mmHg; all had been on three medications, one of which was a diuretic. The objectives were to analyse the reduction in ofce BP, as well 24-hour ABPM.
Preliminary data from this study show that the absolute reduction in ofce BP was 12.5/7.5 mmHg (17.6/8.8 mmHg in patients with available ABPM, n=36) after six months, numerically less than the reductions observed in the Symplicity HTN-1 and Symplicity HTN-2 clinical trials (see Figure 3).29 This is unsurprising given the lower baseline BP in the patient population. Heart rate dropped signicantly from 67 to 63 beats per minute. In 37 % of the patients, antihypertensive medication was reduced during the follow-up period, despite the guidance of the study protocol not to do so. Antihypertensive medication was not increased in any patient. In 51 % of the patients, ofce BP was controlled (dened as <140/90 mmHg) after RDN. Furthermore, there was a substantial reduction in 24-hour ambulatory BP (14.1/6.6 mmHg) (see Figure 3). An increasing body of evidence suggests that reduction of 24-hour ambulatory BP may provide superior cardiovascular risk reduction to ofce BP.30 However, management decisions based on the interpretation of ABPM patterns are more complex than with ofce BP, and suitable educational processes are required.
In summary, although this was a small study and lacked a control group, the data indicated that RDN resulted in a substantial reduction in both ofce and 24-hour ambulatory BP in mild to moderate resistant hypertension. These results will need to be conrmed in a larger study. The SYMPLICITY HTN-4 trial, which is planned to commence enrolment in the latter half of 2013, will address this patient cohort, as well as the broader patient population.