Coronary Occlus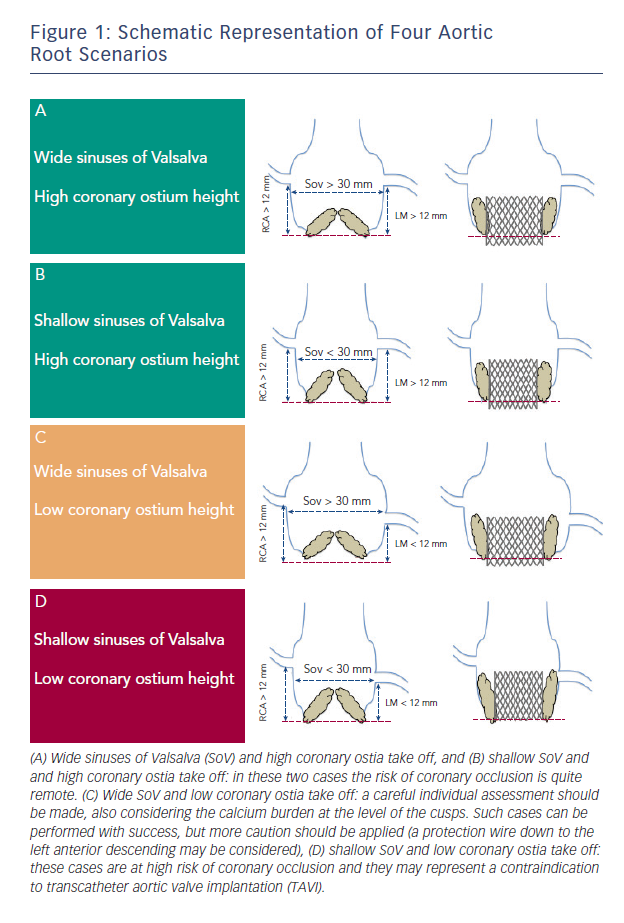 ion
ion
The occurrence of coronary occlusion after TAVI was first described in 2006.9 In contemporary series its incidence has usually been less than 1 %, ranging from 0 % to 4.1 %.2 A recent systematic review showed that reported cases of coronary obstruction following TAVI occurred more frequently in women and patients receiving a balloon-expandable transcatheter heart valve (THV) (SAPIEN, Edwards Lifesciences, Irvine, CA, US), and the left coronary artery was the most commonly involved.6
In native aortic valves, coronary ostia obstruction is related to two main procedural factors: 1) In the vast majority of cases it occurs due to the displacement of a bulky calcified native valve over a coronary ostium; 2) the second option is more hypothetical: it occurs following an obstruction by a portion of the THV frame or the sealing cuff placed directly over a coronary ostium; however, no cases of coronary obstruction related to the struts of the THV frame or to the cuff/leaflets of the transcatheter valve itself have been reported to date.
In both cases there are some anatomic features (narrow sinus of Valsalva, bulky leaflet calcifications, low-lying coronary ostia) that have been involved in its pathogenesis (see Figure 1). In a multicentre study enrolling 44 patients who suffered symptomatic coronary occlusion among a large series of 6,688 TAVIs, Ribeiro and colleagues demonstrated that lower-lying coronary ostium (<12 mm) and shallow sinuses of Valsalva (<30 mm) we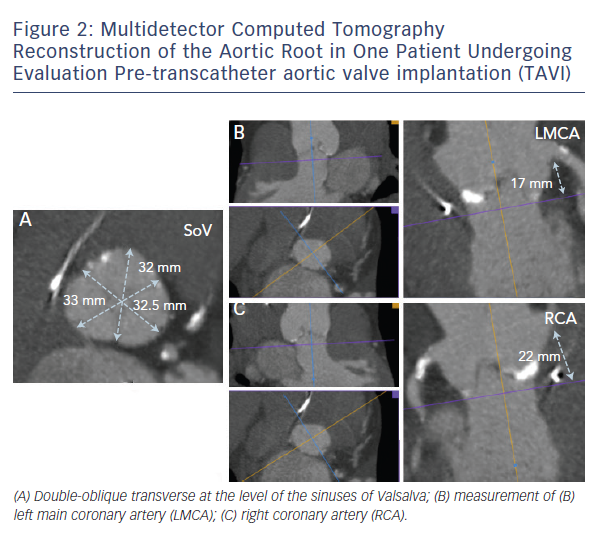
A careful evaluation by means of multimodality imaging can help to highline the bulkiness of the native cusps, the height of the coronary ostia and the dimensions of the sinus of Valsalva.
In daily clinical practice, these evaluations can be made by using multidetector computed tomography (MDCT), which allows for a granular 3D reconstruction of the aortic root, providing extremely reliable assessment of dimensions and calcium distribution (see Figure 2).10,11 3D echocardiogram is also an important tool for aortic root measurements and for pre-procedural assessment of the coronary ostia, but it is limited by its inability of characterise aortic root calcifications.12
When coronary obstruction does occur, clinical presentation is generally characterise by severe hypotension, ST-segment changes and procedural ventricular arrhythmias.6,8 Successful management may require temporary cardiopulmonary support and revascularisation. When the MDCT assessment shows that a certain aortic root anatomy may be prone to develop coronary impairment after valve deployment, a pre-implant balloon v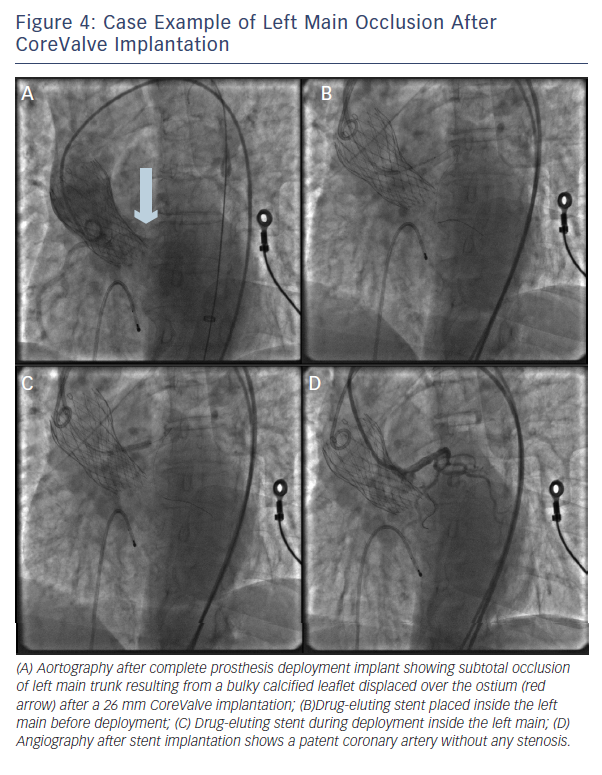 alvuloplasty with simultaneous associated aortography may be useful to ensure the patency of the coronary ostia during balloon inflation; according to this technique, when the balloon is fully inflated, the coronary ostium could be occluded by the calcified cusps crushed against the wall of the sinuses of Valsalva (see Figure 3). In that case, the operator could consider terminating the procedure and considering other options.13 Alternatively, few strategies could be adopted: 1) implanting the prosthesis slightly lower into the left ventricle outflow tract (LVOT), in order to reduce the movement of the cusps toward the coronary ostia and the sinus walls (when the CoreValve prosthesis [Medtronic Inc., Minneapolis, MN, US] is implanted); 2) placing a coronary wire in the periphery of the left anterior descending in order to be ready to restore the patency of the coronary ostia by ballooning and/or stenting the left main, if it should be needed.6 If the patency of the coronary cannot be restored and the haemodynamic is poor, the valve should immediately be snared (CoreValve), or removed from its anatomical position by using an oversized balloon (i.e. SAPIEN prosthesis) and pulled up out into the ascending aorta to allow coronary perfusion to be re-established.14 Recently, Ribeiro and co-workers showed that PCI was the preferred strategy for the treatment of coronary obstruction following TAVI.8
alvuloplasty with simultaneous associated aortography may be useful to ensure the patency of the coronary ostia during balloon inflation; according to this technique, when the balloon is fully inflated, the coronary ostium could be occluded by the calcified cusps crushed against the wall of the sinuses of Valsalva (see Figure 3). In that case, the operator could consider terminating the procedure and considering other options.13 Alternatively, few strategies could be adopted: 1) implanting the prosthesis slightly lower into the left ventricle outflow tract (LVOT), in order to reduce the movement of the cusps toward the coronary ostia and the sinus walls (when the CoreValve prosthesis [Medtronic Inc., Minneapolis, MN, US] is implanted); 2) placing a coronary wire in the periphery of the left anterior descending in order to be ready to restore the patency of the coronary ostia by ballooning and/or stenting the left main, if it should be needed.6 If the patency of the coronary cannot be restored and the haemodynamic is poor, the valve should immediately be snared (CoreValve), or removed from its anatomical position by using an oversized balloon (i.e. SAPIEN prosthesis) and pulled up out into the ascending aorta to allow coronary perfusion to be re-established.14 Recently, Ribeiro and co-workers showed that PCI was the preferred strategy for the treatment of coronary obstruction following TAVI.8
Importantly, PCI was feasible (attempted in 75 % of the patients) and had a success rate of 81.8 % (see Figure 4). Still, urgent coronary artery bypass graft (CABG) or mechanical haemodynamic support were needed in 14 % and 36 % of the patients, respectively.8