Randomized Evidence—PFO Closure versus Medical Therapy
Two devices for percutaneous PFO closure (STARFlex, NMT Medical, Boston, MA, US; Amplatzer PFO Occluder, St Jude Medical, Plymouth, MN) have been investigated in three randomized clinical trials and compared with medical therapy among patients with cryptogenic stroke or embolism.9–11
CLOSURE I Trial
The first randomized trial investigating whether percutaneous PFO closure is effective in the secondary prevention of recurrent ischemic stroke was the multicenter CLOSURE I trial.9 PFO closure was performed with the STARFlex closure device, and medical therapy consisted of either warfarin (target international normalized ratio [INR] 2–3) or acetylsalicylic acid (325 mg/d) as implemented by the treating neurologist. A total of 909 patients with a previous history of stroke or transient ischemic attack (TIA) were included in this study and randomized in a 1:1 fashion to either undergo PFO closure or medical therapy between 2003 and 2008. The primary endpoint of the study was assessed after 2 years and consisted of a composite endpoint of stroke or TIA within 2 years, death of any cause during the first 30 days, and death of neurologic cause after 30 days up to 2 years of follow-up. After 2 years of follow-up, no difference was observed in the primary endpoint between percutaneous PFO closure and medical therapy (adjusted hazard ratio [HR] 0.78, 95 % confidence interval [CI] 0.45–1.35; p=0.37). While the low event rates have been noted, procedural and device specific attributes and complications attracted considerable attention. Effective closure of PFO closure with the STARFlex device was achieved in only 87 % of patients after 2 years of follow-up and device-associated thrombus was as frequent as 1.1 % of patients and considered responsible for recurrent stroke in two patients. Of note, the risk for new onset of atrial fibrillation was almost eightfold increased among PFO closure patients compared with those allocated to medical treatment (5.7 % versus 0.7 %; p<0.001), two-thirds of which have been observed in the first week after the intervention.
PC Trial
The Amplatzer PFO Occluder was investigated in the PC trial, which was initiated in 2000 as a multicenter, randomized clinical trial.10 A total of 414 patients have been recruited and randomized to either PFO closure using the Amplatzer PFO Occluder (n=204) or medical therapy (n=210). After a mean follow-up duration of 845 patient-years in the closure group and 835 patient-years in the medical therapy group, the predefined combined primary endpoint of all-cause death, recurrent stroke, TIA, or peripheral embolism had occurred in seven patients of the closure group compared with 11 patients in the medical therapy group (HR 0.63; 95 % CI 0.24–1.62; p=0.34). Recruitment was protracted over almost 9 years, and the event rate was low in both treatment arms. Of note, a numerical difference in recurrent stroke was observed with an 86 % relative risk reduction in the Amplatzer PFO Occluder compared with the medical therapy group (HR 0.14, 95 % CI 0.02–1.17; p=0.07) after implementing the endpoint definition applied in the RESPECT trial.
RESPECT Trial
The RESPECT trial was conducted between 2003 and 2011 as multicenter, randomized clinical trial to investigate the effectiveness of PFO closure with the Amplatzer PFO Occluder in the secondary prevention of ischemic vascular events. In the overall patient cohort of 980 patients and after a maximum follow-up duration of 8.1 years of follow-up, the primary endpoint recurrent stroke or death within either 30 days after the intervention or 45 days after randomization was observed in nine patients of the PFO closure group compared with 16 patients of the medical therapy group (HR 0.49, 95 % CI 0.22–1.11; p=0.08). While the intention-to-treat analysis provided a numerical difference in the primary endpoint between treatment groups, the per-protocol analysis (HR 0.37, 95 % CI 0.14–0.96; p=0.03) and the as-treated analysis (HR 0.27, 95 % CI 0.10–0.75; p=0.007) favored percutaneous PFO closure over medical therapy. Of note, the treatment effect was particularly pronounced among patients with high-risk PFO criteria as defined by the presence of a substantial shunt (0.8 % versus 4.3 %; p=0.012) or atrial septal aneurysm (1.1 % versus 5.3 %; p=0.016) at baseline. None of the patients had signs of device-related thrombus during echocardiographic follow-up in RESPECT and the PC trial. A nonsignificant twofold increased risk for new onset of atrial fibrillation was noted in the closure group compared with patients receiving medical therapy alone (3.0 % vs 1.5 %; p=0.13), but this was not associated with adverse events.
Randomized Evidence—PFO Closure Devices
Most recently, long-term data from a randomized controlled trial investigating clinical outcomes and device-specific differences of three PFO occluder devices (Amplatzer PFO Occluder, STARFlex, or HELEX, (WL Gore and Associates, Flagstaff, AZ) became available,12 and provided further information for the ongoing debate. A total of 660 patients with previous cryptogenic stroke and PFO were recruited and randomized to closure with one of these closure devices. No differences were observed in technical success between devices; however, significant differences in effective PFO closure rates were observed (Amplatzer versus STARFlex versus HELEX, 98.6 % versus 96.8 % versus 91.8 %; p=0.0012) during follow-up assessment. The primary composite endpoint, recurrent cerebral ischemia, death from neurologic cause, or paradoxical embolism within 5 years after the index procedure was observed in 1.4 %, 6.0 %, and 4.0 % of patients, respectively (p=0.04). Furthermore, there were significant differences in device-associated thrombus formation (0 % versus 5.0 % versus 0.5 %, respectively; p<0.0001) and new-onset of atrial fibrillation (3.6 % versus 12.3 % versus 2.3 %, respectively; p<0.0001) during the followup duration of 5 years.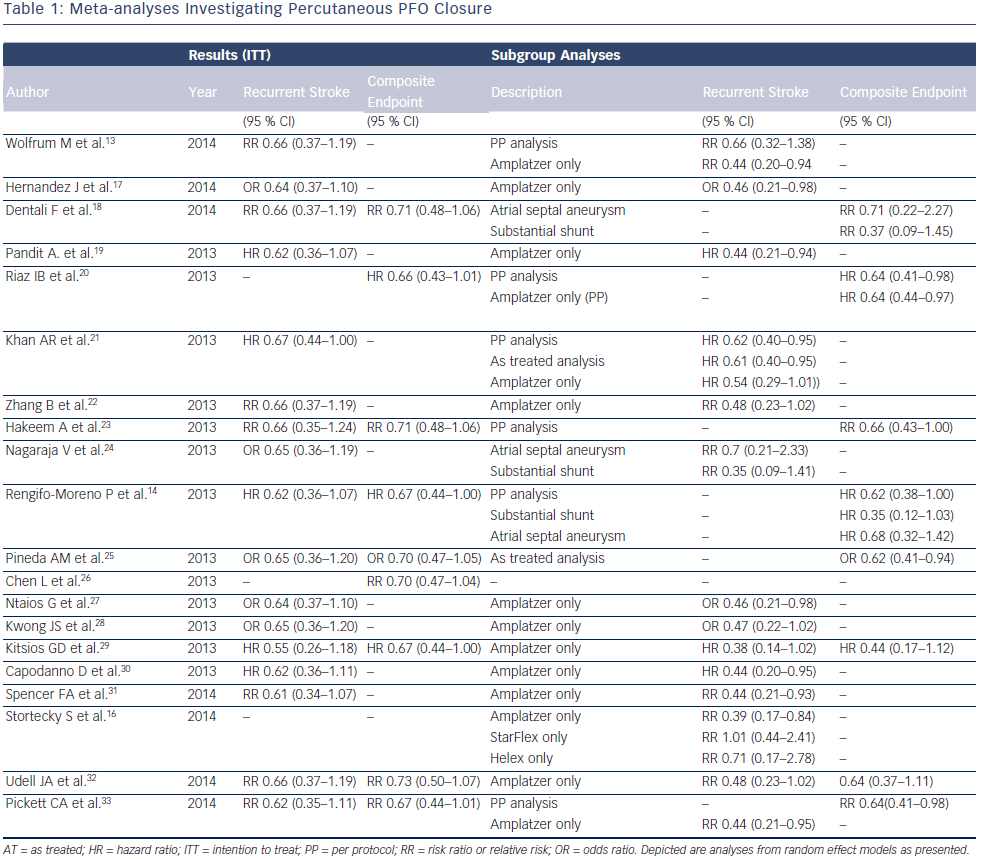
Meta-analyses Summarizing Randomized Evidence
Several meta-analyses with various statistical approaches and different composite endpoints have been published to date, summarizing the available evidence from randomized clinical trials (see Table 1). By combining the results of the three randomized trials investigating PFO closure compared with medical therapy, Wolfrum et al. came to the conclusion that PFO closure does not appear to be superior in patients with cryptogenic stroke or embolism,13 showing a nonsignificant relative risk (RR) reduction of 44 % in the primary intention-to-treat (RR 0.66, 95 % CI 0.37–1.19) and in the per protocol analysis (RR 0.66, 95 % CI 0.32–1.38). Similarly, Kitsios et al. failed to show a significant reduction for the endpoint stroke (HR 0.55, 95 % CI 0.26–1.18); however, a significant effect was observed when analyzing the composite primary outcomes (HR 0.67, 95 % CI 0.44– 1.00). By using the same trial data and in contrast to the previous analyses, Rengifo-Moreno et al. was able to prove a relevant benefit in the reduction of cerebrovascular events (recurrent stroke and TIA; HR 0.59, 95 % CI 0.36–0.97) and furthermore in the combined endpoint of death and vascular events (HR 0.67, 95 % CI 0.44–1.00). Moreover, in a subgroup analysis the authors point towards a potential beneficial effect of percutaneous PFO closure among patients with substantial PFO shunt (HR 0.35, 95 % CI 0.12–1.03), however, this did not reach statistical significance.14 Capodanno et al. reported no beneficial effect for percutaneous PFO closure when compared with medical therapy in the secondary prevention of stroke (HR 0.62, 95 % CI 0.34–1.11).15 However, separating the data according to the PFO device used showed a significant reduction of stroke (HR 0.44, 95 % CI 0.20–0.95) among patients receiving the Amplatzer PFO Occluder, suggesting a device-specific effect on clinical outcomes.
A variation in effectiveness and safety between different devices has been further emphasized after the availability of the long-term data of a randomized head-to-head comparison of the three most frequently used devices.12 Using the data of this head-to-head comparison, potential device specific effects on clinical outcomes were investigated by performing a network meta-analysis (see Table 1).13–33 Overall, the results of this analysis indicated a clinically relevant difference in effectiveness between the Amplatzer and the STARFlex device.16 Accordingly, the probability to be best in preventing recurrent cerebrovascular events was 77.1 % for the Amplatzer, 20.9 % for the Helex, and 1.7 % for the STARFlex PFO closure device, and only 0.4 % for medical therapy alone. Differences in device design and material resulted in a significant reduction of recurrent stroke when using the Amplatzer (RR 0.39, 95 % CI 0.17–0.84), whereas the STARFlex (RR 1.01, 95 % CI 0.44–2.41) and the Helex device (RR 0.71, 95 % CI 0.17–2.78) were not able to show a statistically significant secondary preventive effect. Conversely, the implantation of the Amplatzer device was associated with a twofold increased risk and the STARFlex with a sevenfold increased risk for new-onset atrial fibrillation compared with medical therapy alone.