Mechanical Thrombectomy
Mechanical thrombectomy devices have several mechanisms: some actively fragment thrombotic material before aspiration such as the AngioJet® (MEDRAD, USA), X-Sizer® (Coviden, USA) and Rinspirator™ System (eV3 Inc., USA) whereas others carry out mechanical aspiration only e.g. the TransVascular Aspiration Catheter® (Nipro, Japan) and Rescue™ (Boston Scientific, USA) devices.
The AngioJet system has been assessed in three randomised trials with conflicting results. Initially a single-centre study of 100 patients showed a reduction in infarct size and improved STR compared with PPCI alone;15 however, the larger AngioJet Rheolytic Thrombectomy In Patients Undergoing Primary Angioplasty for Acute Myocardial Infarction (AiMI) trial (480 patients) found no advantage of the AngioJet system in terms of ‘Thrombolysis In Myocardial Infarction’ (TIMI) 3 flow, MBG or STR compared with PPCI alone.16 In fact, final infarct size and MACE rates at 30 days were higher in the thrombectomy group. Subsequently, the Comparison of AngioJet Rheloyic Thrombectomy Before Direct Infarct Artery Stenting With Direct Stenting Alone in Patients with Acute Myocardial Infarction (JETSTENT) trial17 recruited 501 patients with STEMI who were randomised to mechanical thrombectomy before direct stenting or to direct stenting alone. Unlike the AiMI study, this study required patients to have angiographically visible thrombus before they were recruited into the study. The study demonstrated no significant differences between the two groups in STR, TIMI 3 flow, TIMI blush grade 3 or infarct size (as assessed by nuclear scanning). However, the mechanical thrombectomy group had reduced MACE at 6 months and improved 1-year event-free survival rates.17 However, 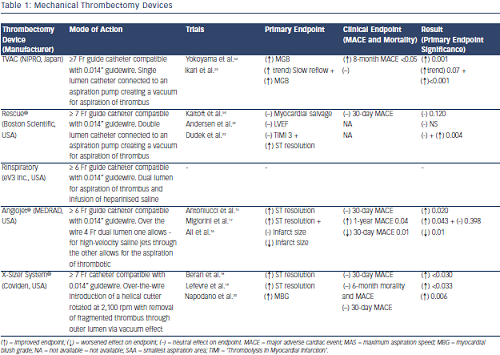 the improved clinical outcomes should be interpreted with caution as with no difference in infarct size or myocardial perfusion between the groups, the mechanism behind the significant clinical benefit is unclear.
the improved clinical outcomes should be interpreted with caution as with no difference in infarct size or myocardial perfusion between the groups, the mechanism behind the significant clinical benefit is unclear.
In the X-Sizer in AMI for Negligible Embolization and Optimal ST Resolution (X AMINE ST) trial, the X-Sizer System® was investigated in 201 patients with STEMI undergoing PPCI. Although the study demonstrated improved STR at 60 minutes post-PCI and demonstrated reduced distal embolisation of debris and lower no reflow rates, it did not demonstrate any significant clinical benefit at 1 and 6 months.18–20 Furthermore, the X-Sizer has been associated with increased rates of coronary artery perforation in other studies.21
In terms of mechanical thrombectomy devices that aspirate thrombus without fragmentation, only the TransVascular Aspiration Catheter has demonstrated positive results with the Rescue® system not associated with any significant improvement in infarct size, MBG or left ventricular ejection fraction in randomised trials.22,23 The VAcuuM asPIration thrombus REmoval (VAMPIRE) study24 was a randomised trial comparing the TransVascular Aspiration Catheter versus PCI alone that showed a small improvement in TIMI flow and MBG. Similar MACE rates were seen at 30 days between the groups; however, a significant reduction in MACE at 8 months in the thrombectomy group was seen, mainly driven by lower revascularisation rates in this group. Importantly no difference in mortality was seen.
Table 1 lists the manual thrombectomy devices currently available as well as the randomised clinical trials investigating their use in STEMI.