Manual Thrombectomy
Manual thrombectomy devices are simpler to use in comparison to mechanical thrombectomy devices. However, due to their mechanism, manual devices cannot extract large amounts of thrombus compared with mechanical thrombectomy devices, which can result in distal embolisation of thrombotic material.25 Manual thrombectomy devices that are currently in clinical use include the Export® Catheter (Medtronic, USA), Hunter (IHT Cordynamics, Spain), Diver® (Invatec, Italy), QuickCat (Spectranetics Inc., USA), Pronto® (Vascular Solutions, USA) and Eliminate (Terumo) among others. Although these devices are similar, they differ in terms of aspiration, lumen size and configuration and there are some differences in the way the thrombus is extracted.
Clinical Trial Data
Manual thrombectomy in PPCI for STEMI has been assessed in a number of clinical trials. The first randomised trial that tested a manual aspiration device was the Randomised Evaluation of the Effect of Mechanical Reduction of Distal Embolisation by Thrombus Aspiration in Primary and Rescue Angioplasty (REMEDIA) study (Diver®). This study randomised 99 patients to PCI with manual aspiration or PCI only.26
This study demonstrated that manual aspiration was associated with significantly better STR and MBG as well as reduced no reflow and distal embolisation. Furthermore, a subgroup analysis demonstrated that thrombus aspiration appeared to be more beneficial in patients with occluded arteries and a higher thrombus burden.26 However, no associated clinical benefit was seen, as the study was underpowered. De Luca et al. found that using manual thrombectomy in patients with anterior STEMI (n=76), demonstrated better post-procedural MBG and better STR at 90 minutes.27 However, again, these findings were not translated into improved clinical outcomes because the study was underpowered. Similar findings were seen in larger studies: Polish-Italian-Hungarian RAndomized ThrombEctomy (PIHRATE) trial (196 patients) and Dethrombosis to Enhance Acute Reperfusion in Myocardial Infarction (DEAR-MI) (Pronto catheter, 148 patients) with again no improved clinical outcomes.28,29 However, the translation of improved procedural outcomes into better clinical outcomes was achieved with the publication of the Thrombus Aspiration During Percutaneous Coronary Intervention in Acute Myocardial Infarction (TAPAS) trial.
TAPAS
The majority of randomised trials have shown that manual thrombectomy is associated with improved MBG, STR and TIMI flow. However, until TAPAS, most of these studies were not powered to detect a clinical benefit. The TAPAS study was a single-centre randomised trial that randomised 1,071 patients with STEMI in a 1:1 fashion to manual aspiration using the Export catheter or PCI alone.30 Patients in the thrombectomy arm had higher MBG, improved STR and fewer pathological Q-waves. Significantly, these beneficial effects on reperfusion resulted in fewer clinical events at both 30 days (reduced mortality and re-infarction) and a significant reduction in mortality at 1 year.31
After TAPAS, other trials have corroborated the benefits of manual thrombectomy. In the Thrombectomy Wit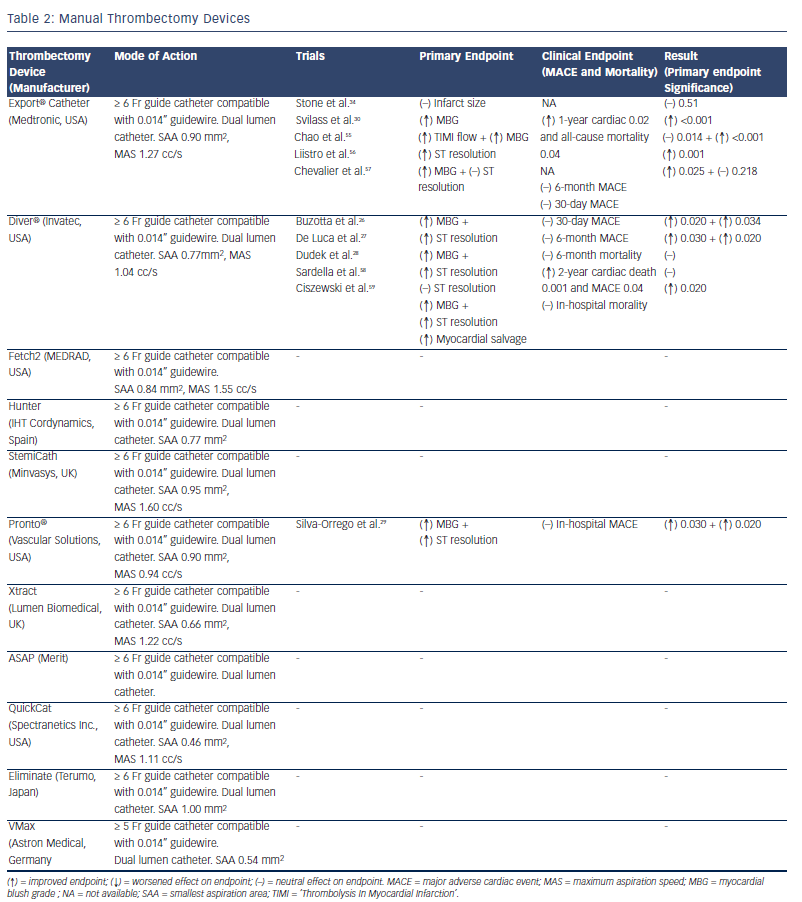 h export Catheter in Infarct- Related Artery During Primary Percutaneous Coronary Intervention (EXPIRA) trial, 175 patients were recruited with MBG, STR and microvascular obstruction (MVO) as primary endpoints. This trial was the first study to assess thrombectomy use with MVO as a primary endpoint.32 The study demonstrated that manual thrombectomy significantly improved both MBG and STR, reduced MVO and final infarct size (as assessed by cardiac magnetic resonance [CMR]) and was associated with reduced cardiac mortality and MACE at 24-month follow-up.32
h export Catheter in Infarct- Related Artery During Primary Percutaneous Coronary Intervention (EXPIRA) trial, 175 patients were recruited with MBG, STR and microvascular obstruction (MVO) as primary endpoints. This trial was the first study to assess thrombectomy use with MVO as a primary endpoint.32 The study demonstrated that manual thrombectomy significantly improved both MBG and STR, reduced MVO and final infarct size (as assessed by cardiac magnetic resonance [CMR]) and was associated with reduced cardiac mortality and MACE at 24-month follow-up.32
However, unlike TAPAS and other single- centre trials, multi-centre studies have mostly been negative. The INFUSE-AMI trial (which was a Factorial, Randomized, Multicentre, Single-Blind Evaluation of Intracoronary Abciximab Infusion and Aspiration Thrombectomy in Patients Undergoing Percutaneous Coronary Intervention for Anterior ST-Segment Elevation Myocardial Infarction) recruited 452 patients presenting early with large anterior STEMI undergoing PPCI. They were randomised in a 2 x 2 factorial design to bolus intracoronary abciximab versus no abciximab and to manual aspiration thrombectomy versus no aspiration.33 Here, manual thrombus aspiration was not effective in reducing infarct size as assessed by CMR or MACE at 30 days.34 Importantly, and most significantly, the recently published Thrombus Aspiration in Myocardial Infarction (TASTE) study is the largest study performed to date and was also negative for clinical improvement. This was a multicentre, prospective, randomised controlled trial (RCT), which randomised 7,244 patients with STEMI undergoing PPCI to manual thrombus aspiration versus PCI alone, with the primary endpoint of 30-day mortality. Manual devices used included Eliminate, Export and Pronto. Although there was a trend towards a reduction in rates of re-infarction at 30 days in the thrombectomy group, there was no significant difference in 30-day mortality between the two groups.35 In addition, there were no significant differences in rates of stroke, heart failure, left ventricular function or stent thrombosis between the two groups.35
Table 2 lists the manual thrombectomy devices currently available as well as the randomised clinical trials investigating their use in STEMI.