Prevalence of Patent Foramen Ovale in the General Population
A number of observational studies, both in vivo and pathological, have indicated a variable prevalence. In 1984 Hagen et al.2 demonstrated from 965 autopsy specimens an overall prevalence of 27.3 %, which decreased with age; 34.3 % in the first three decades, 25.4 % during the 4th to 8th decades and 20.2 % during the 9th and 10th decades. The size ranged from 1 mm to 19 mm and was inversely related to age. Fifteen years later Meissner et al.3 found a similar rate of PFO in vivo. Using transoesophageal echocardiography (TOE) without contrast in 585 subjects over 45, they demonstrated a prevalence of 26 % of PFO. Furthermore, increased numbers within families has suggested a genetic basis for the failure of closure.4
Patent Foramen Ovale in Cryptogenic Stroke
A cryptogenic stroke (CS) occurs when no apparent cause can be found despite extensive investigation. The concept was initially developed for the stroke Data Bank by the US National Institute of Neurological and Communicative Disorders and Stroke5 and has been shown to account for up to 40 % of all strokes .6
Paradoxical embolism (PE) through a PFO has been implicated in such strokes since the 19th century, when the distinguished pathologist Julius Cohnheim performed an autopsy on a young lady who had succumbed to a large stroke.7 He noted venous thrombus and a large PFO, and hypothesised that a clot had formed in the lower limb veins and then traversed the PFO and onwards to the cerebral circulation.
In the 1950s case reports appeared in the medical literature of thrombus trapped in PFOs.8–10 However, it was not until the 1980s, with the advent of wider spread echocardiography, that researchers began searching for evidence to support a link between ischaemic strokes or transient ischaemic attacks and PFOs.
In 1988 two observational studies were published suggesting an association between PFO and cryptogenic stroke.11,12 At first, Lechat et al. demonstrated that those under 55 with CS had a 54 % prevalence of PFO, while Webster et al. found 50 % prevalence in their patients with ischaemic stroke or transient ischaemic attack (TIA). Both studies utilised contrast echocardiography to detect PFOs. A number of other studies have corroborated the link, even extrapolating to older patients with CS.13,14
An associated atrial septal aneurysm (ASA), when the septum moves more than 1 cm during the cardiac cycle, has been shown to have a synergistic effect to increase risk.15 Mas et al.16 investigated the role of PFO and ASA in recurrent stroke. They followed 581 patients (under the age of 55) with a history of CS treated with aspirin – a TOE demonstrated an atrial septal abnormality in 48 %. After four years the rate of recurrent stroke was 6.2 % in those without an atrial septal abnormality, 5.6 % in the group with PFO alone and 19.2 % in the group with both PFO and ASA. A further meta-analysis of 15 case control studies17 revealed that stroke under the age of 55 was associated with either PFO or ASA alone or PFO and ASA together.
Not all studies found such a clear association. In the Patent Foramen Ovale in Cryptogenic Stroke study (PICCS),18 which was a TOE substudy of the Warfarin-Aspirin Recurrent Stroke study (WARSS), there was no relationship between recurrent stroke and PFO, regardless of size.
The first case series of catheter-based permanent PFO closure was published in 1992.19 Bridges et al. used a doubl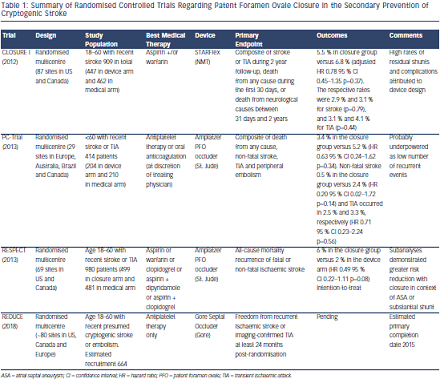 e umbrella device on 36 patients with presumed complications of PE (31 ischaemic strokes, 25 TIAs, four systemic arterial emboli and two brain abscesses). As well as a high rate of shunt closure and a very low rate of complication, they did not see any post-procedural events during a follow-up of 8.4 months.
e umbrella device on 36 patients with presumed complications of PE (31 ischaemic strokes, 25 TIAs, four systemic arterial emboli and two brain abscesses). As well as a high rate of shunt closure and a very low rate of complication, they did not see any post-procedural events during a follow-up of 8.4 months.
Numerous small non-randomised series followed, brought together in a pooled analysis of 16 studies20 (10 of transcatheter closure and six of medical therapy) that showed the one-year rate of recurrent stroke in medically treated patients was 3.8–12.0 % compared with 0.0–4.9 % in those who underwent device closure. On the basis of these observations it was predicted that a randomised trial would prove that PFO closure was preferable to medical therapy to prevent recurrent cryptogenic stroke.
Recently there have been three published randomised trials – Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/ or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale (CLOSURE I),21 the Randomized clinical trial comparing percutaneous closure of patent foramen ovale using the Amplatzer PFO Occluder with medical treatment in patients with cryptogenic embolism (PC-Trial)22 and the Randomised Evaluation of recurrent Stroke comparing PFO closure to Established current standard of Care Treatment (RESPECT) trial23 – with a further one currently recruiting (GORE HELEX™ Septal Occluder and Antiplatelet Medical Management for Reduction of Recurrent Stroke or Imaging-Confirmed TIA in Patients With Patent Foramen Ovale [REDUCE]), designed to determine the effectiveness of PFO closure as secondary prevention in cryptogenic stroke (see Table 1).