Pathophysiological Effects of Sleep-disordered Breathing
Although OSA is traditionally thought to accelerate cardiovascular deterioration, whereas CSA is predominantly a marker of severity of HF, both types of SDB share common deleterious pathophysiological consequences. However, there is evidence that CSA may in some cases be adaptive in HF.
In OSA, apnoea is accompanied by negative intrathoracic pressure as the respiratory muscles attempt to inspire against a collapsed airway. This enhances venous return to the right heart, increasing pre-load, which increases the work of the failing heart and pushes the septum to the left, further embarrassing LV function. In addition, negative intrathoracic pressure opposes contraction of the ventricular free wall, effectively increasing afterload. Recurrent episodes of apnoea and hypopnoea lead to arousals and sympathetic nervous system stimulation, known to be maladaptive in HF, and swings in heart rate and blood pressure.13 High concentrations of urinary catecholamines are found in patients with OSA14 and sleep quality is disrupted, often resulting in day-time somnolence and increased risk of road traffic accidents.15 In patients with OSA there is evidence of abnormal endothelial function – the vasodilatory response to nitric oxide is blunted and expression of the vasoconstrictors endothelin-1 and angiotensin II increased.14,16 OSA is associated with hypertension, coronary artery disease, stroke and arrhythmias, probably due to both shared risk factor profiles and the mechanisms described. These maladaptive mechanisms affect both systolic and diastolic cardiovascular function.
Perhaps unsurprisingly, patients with OSA and HF have a significantly increased mortality compared with those without OSA, particularly with co-existent ischaemic heart 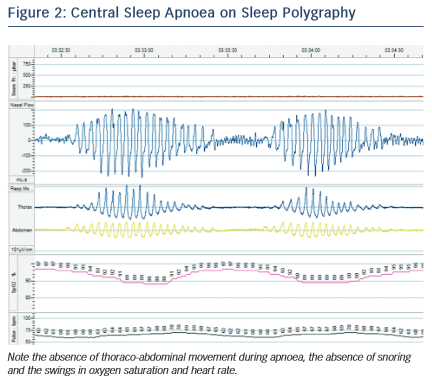 disease.17 In the Sleep Heart Health Study, a large community-based cohort observational study, the presence of severe OSA was associated with more than twice the all-cause mortality risk over 8 years of follow-up.18 OSA was also associated with a 58 % increase in risk of developing HF de novo.19 Patients with OSA and HF have an increased risk of sudden cardiac death, malignant arrhythmia and the need for implantable defibrillator therapy, particularly during the night; in patients with HF and either CSA or no SDB, malignant arrhythmias and defibrillator therapies occur predominantly during the day.20,21
disease.17 In the Sleep Heart Health Study, a large community-based cohort observational study, the presence of severe OSA was associated with more than twice the all-cause mortality risk over 8 years of follow-up.18 OSA was also associated with a 58 % increase in risk of developing HF de novo.19 Patients with OSA and HF have an increased risk of sudden cardiac death, malignant arrhythmia and the need for implantable defibrillator therapy, particularly during the night; in patients with HF and either CSA or no SDB, malignant arrhythmias and defibrillator therapies occur predominantly during the day.20,21
CSA is also associated with a worsened prognosis in HF. In one study, the presence of any CSA (including mild disease) was associated with a significantly shorter mean survival in patients with EF <45 %: 45 months versus 90 months.22 This has traditionally been attributed to CSA being a marker of more severe cardiac dysfunction. However, there are reasons to believe that CSA itself may accelerate HF. Patients with CSA experience episodes of hypoxaemia and swings in heart rate and blood pressure, in a similar way to OSA (see Figure 2). CSA is associated with higher sympathetic nervous system activity, known to be maladaptive in HF,23 although there is debate as to whether this is due to the CSA itself or the underlying HF.24,25 A possible role for CSA in driving enhanced sympathetic activity is suggested by the finding that administered oxygen and continuous positive airway pressure (CPAP) both ameliorate sympathetic tone.26 The episodes of hyperventilation in CSA may also increase demands on the failing heart. However, in contrast to OSA, there is no marked negative intrathoracic pressure during apnoeas and hypopnoeas, as the respiratory muscles are not stimulated, so many of the haemodynamic effects of this (such as changes in ventricular pre- and after-load) are absent.
This view of CSA has been challenged recently by the unexpected findings of the SERVE-HF trial.8 In this randomised study, treatment of patients with HF and predominant CSA with adaptive servo- ventilation (a non-invasive ventilation technique known to ameliorate the peaks and troughs of ventilation in CSA very effectively) had no impact on the primary combined endpoint of time to death, life- saving cardiovascular intervention or unplanned HF hospitalisation, but did increase the risk of all-cause and cardiovascular mortality compared with controls (hazard ratio for death from any cause, 1.28, 95 % confidence interval [CI] 1.06–1.55; p=0.01). This surprising result raises the possibility that CSA may in fact be protective in HF and there are possible explanations for this, as described by Naughton in 2012.27 Indeed, in patients without cardiovascular disease, the Sleep Heart Health Study found that CSA was not associated with increased mortality.18 The increased mortality seen in patients with HF and CSA may be partly related to the difficulty in identifying an appropriate control group (as those with CSA tend to have more severe cardiac dysfunction even within the inclusion criteria of the studies).
There are several proposed mechanisms for the potential cardio- protective effects of CSA. The episodes of hyperventilation lead to end-tidal volume increases of 400 ml on average.28 This increases oxygen storage in the lung, reduces hypoxaemia in the presence of pulmonary oedema and impaired gas exchange and improves lung compliance in a similar way to CPAP therapy. The hyperventilation phase of CSA has been shown to reduce sympathetic and increase vagal tone, and the elevated sympathetic tone seen in those with HF and CSA relates more closely to the severity of HF than of CSA.25 Importantly, the episodes of hyperventilation induce a respiratory alkalosis. Hypocapnia has been shown to preserve myocardial function in the presence of hypoxia in dogs29 and alkalosis results in better myocardial performance during hypoxia in vitro.30 Hypocapnia and alkalosis also increases the oxygen- carrying capacity of haemoglobin, according to the Bohr and Haldane effects. As hypercapnia and acidosis are a frequent finding in acute decompensated HF, this may have a protective role. Swings in intrathoracic pressure with hyperventilation may also augment cardiac output via pump-like variations in pre- and after-load. In addition, hyperventilation is thought to reverse oedema-induced bronchoconstriction.31 During apnoeic episodes in CSA there is also slightly elevated intrathoracic pressure, which may prevent alveolar collapse.32 Furthermore, recurrent episodes of hypoxaemia may stimulate erythropoiesis and it is postulated that alternating high and low workload may reduce respiratory muscle fatigue and improve oxygenation compared with constant effort.33 The effect of adaptive servo-ventilation (ASV) on these protective mechanisms may, at least in part, explain the surprising increase in cardiovascular mortality found in the SERVE-HF trial.