Central Sleep Apnoea
Following successful trials of CPAP in OSA and HF, the CANPAP trial sought to evaluate its use in those with CSA.51 This study randomised 258 patients with HF and CSA to CPAP or optimal medical therapy only with a mean follow up of 2 years. CPAP was effective at improving AHI (–21 ± 16 versus –2 ± 18 per hour; p<0.001) and nocturnal oxygen saturation, as well as plasma noradrenaline concentration, LVEF and 6-minute walk distance. However, CPAP had no overall influence on rates of hospitalisation and death compared with controls. A post- hoc analysis did demonstrate a survival advantage in those treated with CPAP in whom AHI was supressed to below 15/hour, raising the possibility that more efficacious ventilatory techniques may provide survival benefit to the wider population with HF and CSA.52
A more complex non-invasive ventilatory modality is ASV. In this mode, the ventilator automatically adjusts the degree of respiratory support such that more inspiratory positive airway pressure (IPAP) is provided during episodes of hypopnoea and apnoea and less during hyperpnoea. It also provides positive end expiratory pressure (PEEP)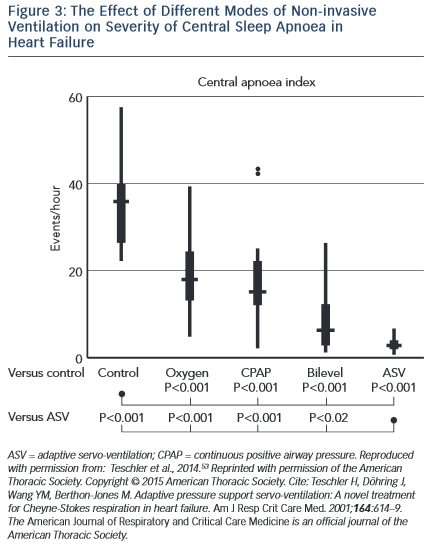 as in CPAP and mandatory breaths during apnoeas. It is therefore potentially effective in both CSA and OSA.
as in CPAP and mandatory breaths during apnoeas. It is therefore potentially effective in both CSA and OSA.
Several studies have demonstrated benefits of ASV on surrogate endpoints in CSA and HF. Teschler and colleagues showed that ASV was significantly more efficacious at suppressing CSA than CPAP, bi-level positive airway pressure (BiPAP) or oxygen therapy (see Figure 3).53 In an observational study, Oldenburg and colleagues found that 6 months of ASV therapy in those with HF and CSA almost eradicated CSA, from 37.4 ± 9.4/h to 3.9 ± 4.1/h (p=0.001) and was associated with markedly improved cardiopulmonary exercise test parameters, increased LVEF (28.2 ± 7 % to 35.2 ± 11 %; p=0.001) and reduced N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) concentration.54 These results were largely replicated by Hastings et al.55 Similar improvements were found in those with HF with preserved EF (HFPEF).56 Reductions in sympathetic nervous activity have also been demonstrated with ASV therapy.57 Furthermore, registry data have revealed a significantly longer time to first implantable cardioverter defibrillator (ICD) therapy or ventricular arrhythmia in patients with HF, CSA and an ICD treated with ASV compared with those not treated58. Meta-analysis of trials suggested an overall reduction in AHI, improved exercise capacity, LVEF and quality of life measures with ASV treatment compared with controls.59 However, these trials were not powered to detect changes in harder clinical endpoints.
SERVE-HF is the first large-scale and longer-term randomised trial to report the effect of ASV on clinical outcomes.8 This multi-centre trial enrolled 1,325 patients who were randomised to ASV in addition to optimal medical therapy or optimal medical therapy only for a median of 31 months. The trial was powered for the combined endpoint of all-cause death, unplanned hospitalisation for worsening HF, heart transplantation, cardiac arrest or appropriate ICD therapy. As discussed above, ASV did not influence the primary end-point but was associated with a significant increase in both all-cause and cardiovascular mortality. Most of the excess mortality related to sudden death, presumably from cardiac arrhythmia, and not only during the night. Based on this study, the largest of its kind in HF and SDB, and despite the beneficial effects demonstrated previously, ASV cannot be recommended for those with CSA and HF.
In the randomised CHF-HOT study of 97 patients with CSA and HF, overnight oxygen therapy reduced AHI and improved LVEF and New York Heart Association (NYHA) grades,60 but the effect on clinical outcomes is unknown. Inhaled carbon dioxide is also effective at reducing AHI, but not arousals or sympathetic nervous activity.61
CRT produces a significant reduction in AHI in appropriately selected patients,62 presumably due to improved cardiac output, reduced pulmonary congestion and ameliorated sympathetic tone. Research is underway to determine whether improvement in CSA with CRT is associated with normalisation of the hypercapnic ventilatory response (NCT02203383). It is unknown whether the presence of CSA should be a factor when considering a patient for CRT.
A novel therapy for CSA is phrenic nerve stimulation. This involves the implantation of a pacemaker-like device with a lead that communicates with the phrenic nerve via the left pericardiophrenic or right brachiocephalic vein. The device stimulates the phrenic nerve when no intrinsic impulse is detected for a specified time period and this stimulates a breath, preventing the hypercapnia, which triggers the cycles of Cheyne-Stokes respiration. Early research suggests a significant improvement in AHI, oxygenation and arousals, but the impact on prognosis is unknown.63 Further research is underway.