Treatment
Although, recent clinical registries3–8,11 have suggested the majority of patients admitted with PO rapidly improve as a result of conventional IV therapies, treatment of PO remains largely opinion-based as there is a general lack of good evidence to guide therapy.27 Moreover, most of the trial data are derived from an AHF population that does not resemble PO patients, and thus the data are not entirely generalisable to the PO clinical profile.
Although multicenter, randomized, controlled trials have been conducted in patients with AHF, none of studied agents showed simultaneous benefit for symptomatic relief, 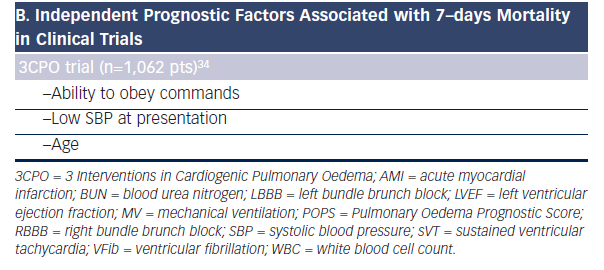
In clinical practice, acute management of PO is based on IV opiates, diuretics, vasodilators, inotropes, and MV. In approximately 70–80 % of cases, PO patients are treated with combinations of IV therapies.6,11 Data from recent registries11 showed there is a specific pattern of utilisation of IV therapies (i.e. high doses for short duration), which may explain the patients’ in-hospital course and adverse outcomes.
Morphine
Morphine is commonly used in the treatment of PO, despite the lack of evidence supporting its efficacy. Morphine is given to cause systemic vasodilatation and alleviate the anxiety related to dyspnea (i.e. ‘air hunger’). In vivo experiments have confirmed that IV morphine results in significant reduction in systemic vascular resistance (SVR) and venous return.37,38 Morphine may induce respiratory depression39 that may worsen hypoxemia in a patient already hypoxicemic and altered cerebral perfusion, leading to intubation. Furthermore, data from Acute Coronary Syndrome (ACS) registries40 and from HF registries41 suggest that morphine therapy was associated with an increase in mortality, need for intensive care unit (ICU) admission, and intubation. The use of IV diuretics and vasodilators can decrease pulmonary congestion and avoid morphine’s adverse effects on respiratory drive. Morphine may be useful in some patients with PO in the setting of myocardial ischemia when analgesia is required, as morphine reduces anxiety and relieves the pain.
Diuretics
Intravenous loop diuretics are an essential component of PO treatment, and recent guidelines27 consider IV diuretics as first-line therapy. Analysis of recent registries (see Table 1) shows that IV diuretics are administered to approximately 90 % of patients who are hospitalized for PO. Furosemide was the most commonly used diuretic. Loop diuretics inhibit reabsorption of NaCl and produce natriuresis and diuresis. The diuretic effect occurs 35–45 minutes after IV administration. They act by inhibiting the renal Na/2Cl/K co-transporter in the luminal membrane of the thick ascending limb of loop of Henle, which is responsible for the reabsorption of 35 % of filtered sodium.42 Of note, furosemide inhibits the same Na/2Cl/K co-transporter in alveolar epithelial cells altering transepithelial chloride secretion and increasing alveolar fluid clearance and oedema resolution.23
Furthermore, IV furosemide produces direct venodilation, an effect that can be seen as rapidly as 2–5 minutes after administration. The direct venodilation was inhibited by local indomethacin administration but not by blockade of nitric oxide (NO) synthesis, indicating that the direct vascular venodilation is dependent on local prostaglandin but not on NO production.43 The rapid reduction in venous return produced by furosemide usually occurs before diuresis and may be therapeutically relevant for obtaining symptomatic improvement in PO. However, the net venodilatory effect of furosemide is difficult to assess, since the decrease of circulating volume produced by furosemide is at the cost of neurohormonal activation.44 Historically, direct venodilation has been one argument for IV diuretic use in PO since the main pathophysiology of PO is represented by fluid redistribution rather than overall fluid accumulation. The efficacy of loop diuretic use is also balanced by limitations of diuretic resistance, neurohormonal activation, and worsening renal function (WRF).
One major concern with use of IV loop diuretic is the phenomenon of nephron adaptation, which occurs in patients previously exposed to long-term use of oral furosemide. Chronic furosemide treatment may induce ‘nephron adaptation’, respectively a reactive increase in active transcellular sodium transport capacity in the tubular segments situated distally to the site of action of furosemide.45 This may result in a reduced natriuretic response at conventional IV diuretic doses45,46 and possibly explain utilisation of higher doses of IV diuretics in PO patients.
There are substantial variations in clinical practice concerning dosing, mode of administration, and duration of therapy, since there is very little Level A, Class I evidence available. A prospective trial comparing four strategies (high versus low dose; bolus versus continuous administration) showed no significant differences in terms of dyspnea improvement or 60-day outcomes.47 However, the high-dose strategy was associated to a trend with a trend toward greater dyspnea improvement at the cost of transient WRF that did not appear to have long-term consequences. Current guidelines27 recommend as the first IV furosemide dose to be 2.5 times the existing oral dose in PO patients already taking diuretic.