In Symplicity HTN-34, blinding of patients through the use of a sham procedure and wider use of ambulatory blood pressure measurement balanced and limited the differential impact of the Hawthorne, white coat, placebo and regression to the mean effects in both treatment arms. This disclosed to the world the true size of blood pressure decrease attributable to RDN, at least in patients meeting the Symplicity criteria; it was less than 3 mmHg systolic based on ambulatory blood pressure monitoring (Table 1). Sham-procedure is however not feasible in clinical practice but was required by FDA in the Symplicity HTN-3 Study to overcome all the pitfalls in hypertension research mentioned above in order to investigate whether RDN has a true blood pressure lowering effect and the procedure may be characterised as “evidence based medicine”. Taken together with another four prospective and randomised clinical trials published or presented in 2014, discussed below, RDN as of today obviously does not fulfill these criteria and should not be used outside research protocols.
For all aforementioned reasons, and in view of the complexity and multifactorial character of hypertension, the failure of RDN to normalise or substantially reduce blood pressure in all patients with apparently resistant hypertension was a reasonable working hypothesis for us, even before the Medtronic announcement that Symplicity HTN-3 had failed to meet its primary endpoint (http://www.tctmd.com/show. aspx?id=123265 ). We32–34 and others19,28 had predicted that the true effect of RDN might have been overestimated and may considerably shrink in properly designed studies.19,29 In particular, in preliminary analysis of the European Network COordinating research on Renal Denervation (ENCOReD) network,35 we were struck by the imbalance between the 17.6 mmHg decreases in office blood pressure, vs only 5.9 mmHg for 24-h ambulatory blood pressure.
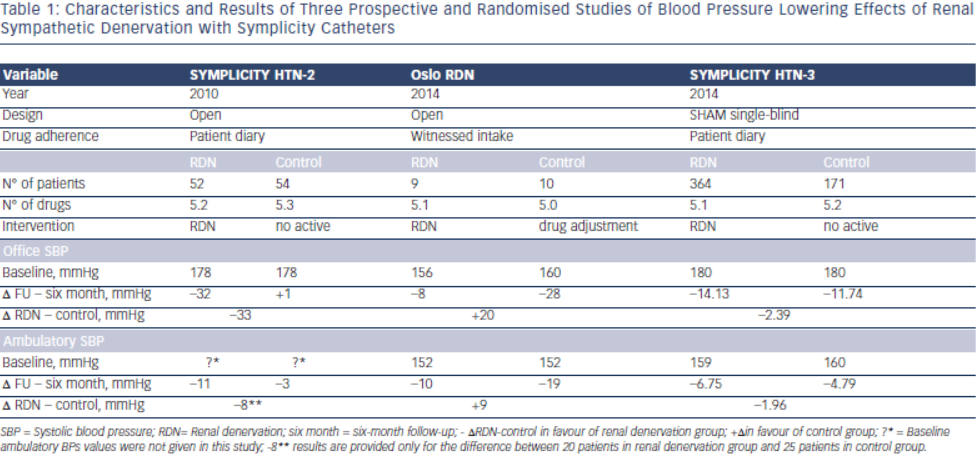 The ENCOReD site in Oslo, with longstanding traditions for randomised research in hypertension36, applied a simple and practical way to deal with pitfalls in the recruitment of patients with resistant hypertension. After extensively ruling out secondary hypertension, and improving drug treatment in the run-in phase, patients had to qualify for the RDN protocols by having elevated daytime ambulatory blood pressures after witnessed oral intake of their prescribed blood pressure medication.33 This was a convenient way to identify the true treatment-resistant hypertensive patients and to exclude patients with white coat hypertension or those non-adherent patients whose blood pressure normalised after witnessed drug intake. Meanwhile a centre in Germany37 published a small but well documented series of patients whose blood pressure remained unchanged after RDN. We were thus not surprised when the Oslo activity found no change in either office or ambulatory blood pressures following RDN, first in an open series of six patients33 and later followed by a randomised study, the Oslo-RDN trial.38 Patients who were randomly assigned to further improvement of drug treatment guided by noninvasive haemodynamic monitoring had normalised blood pressures. In contrast, patients exposed to RDN experienced only a small and probably partly placebo-induced fall in office and ambulatory blood pressures (see Table 1). The decreases averaged 20 mmHg more for office and 9 mmHg more for ambulatory systolic blood pressure in the haemodynamically guided drug treatment group compared to the RDN group.
The ENCOReD site in Oslo, with longstanding traditions for randomised research in hypertension36, applied a simple and practical way to deal with pitfalls in the recruitment of patients with resistant hypertension. After extensively ruling out secondary hypertension, and improving drug treatment in the run-in phase, patients had to qualify for the RDN protocols by having elevated daytime ambulatory blood pressures after witnessed oral intake of their prescribed blood pressure medication.33 This was a convenient way to identify the true treatment-resistant hypertensive patients and to exclude patients with white coat hypertension or those non-adherent patients whose blood pressure normalised after witnessed drug intake. Meanwhile a centre in Germany37 published a small but well documented series of patients whose blood pressure remained unchanged after RDN. We were thus not surprised when the Oslo activity found no change in either office or ambulatory blood pressures following RDN, first in an open series of six patients33 and later followed by a randomised study, the Oslo-RDN trial.38 Patients who were randomly assigned to further improvement of drug treatment guided by noninvasive haemodynamic monitoring had normalised blood pressures. In contrast, patients exposed to RDN experienced only a small and probably partly placebo-induced fall in office and ambulatory blood pressures (see Table 1). The decreases averaged 20 mmHg more for office and 9 mmHg more for ambulatory systolic blood pressure in the haemodynamically guided drug treatment group compared to the RDN group.
In the absence of solid evidence of efficacy, how can we explain the uncontrolled deployment of RDN in Europe and worldwide (with the notable exception of the US where RDN remained an investigational procedure)? Of course, publications of the Symplicity studies and of multiple observational studies, and enthusiastic reports, editorials and reviews1–3,39,40 had a substantial impact, and the lack of strict rules for introduction of device-based therapies in Europe facilitated the large-scale implementation of the technique. However, this phenomenon would have remained limited without the huge promotion by device-producing industry. Medical journals were swamped by reviews and meta-analyses showing the powerful blood pressure lowering effects as recorded in observational studies and in the single available randomised study, Symplicity HTN-2. Comments pointing out the defects and inconsistencies in such meta-analysis encountered great delay in getting published.41 Many never questioned whether RDN should be implemented, but when it should start in an institution. By all means, the purpose was to disseminate the enthusiasm for RDN from the technically-oriented invasive radiologists and cardiologists who usually had little interest or experience in the treatment of hypertension to the “hypertension establishment.” The European Society of Hypertension issued specific guidelines,42,43 but maintained reservations that more data was needed, and eventually it had to be proven that RDN would lower morbidity and mortality before being generally accepted in the treatment of true or apparent treatment- resistant hypertension.