Aortic valve
Severe symptomatic aortic stenosis (AS) bears a dismal prognosis. The mean survival is 2.0 to 4.7 years after the onset of angina, 0.8 to 3.8 years after the onset of syncope and 0.5 to 2.8 years after the onset of congestive heart failure.1 Surgical aortic valve replacement (SAVR) is the mainstay of treatment for these patients.2 In the last decade transcatheter aortic valve replacement (TAVR) has become an accepted alternative in patients for whom surgery would impart a high or prohibitive risk of mortality or mo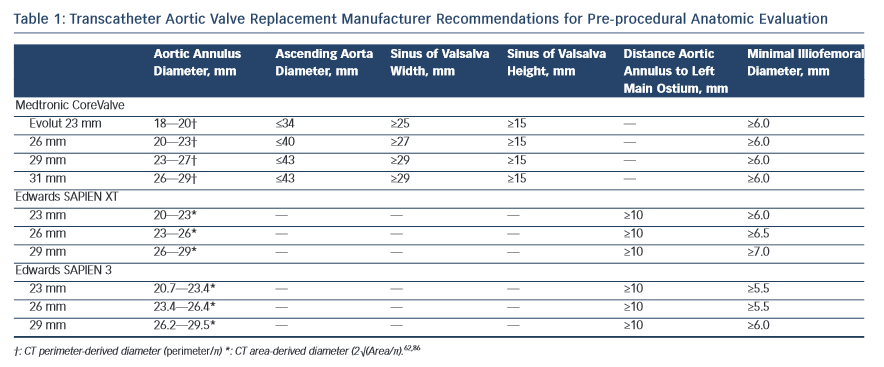 rbidity. TAVR does not require openheart surgery. Instead, bovine or porcine pericardial prosthetic leaflets are mounted on a metal frame, which is delivered using a catheter via an endovascular or transapical route. The CoreValve US Pivotal Trial showed absolute mortality benefit of TAVR over SAVR of 4.9 % in high-risk patients (P<0.001 for non-inferiority, P=0.04 for superiority) at one year follow up.3 In the Placement of Aortic Transcatheter Valves 1 trial (PARTNER 1 trial), the absolute mortality benefit in TAVR versus SAVR was of 5.4 % at five years (P=0.76).4
rbidity. TAVR does not require openheart surgery. Instead, bovine or porcine pericardial prosthetic leaflets are mounted on a metal frame, which is delivered using a catheter via an endovascular or transapical route. The CoreValve US Pivotal Trial showed absolute mortality benefit of TAVR over SAVR of 4.9 % in high-risk patients (P<0.001 for non-inferiority, P=0.04 for superiority) at one year follow up.3 In the Placement of Aortic Transcatheter Valves 1 trial (PARTNER 1 trial), the absolute mortality benefit in TAVR versus SAVR was of 5.4 % at five years (P=0.76).4
The dimensions of the aortic valve complex are critical for sizing of device (see Table 1 and Figure 1). The aortic annulus, sinuses of Valsalva, ascending aort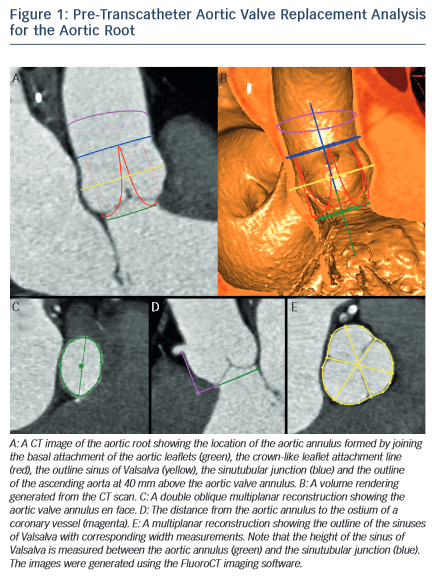 a, coronary arteries ostia and any bypass grafts should be assessed in all patients considered for TAVR.5 While 2D echocardiography provides valuable haemodynamic information and is readily available, a MSCT-based analysis of the aortic root and vascular access sites has become a sine qua non step in the pre-operative evaluation of patients for TAVR.6 Device sizing using multi-slice computer tomography (MSCT) has been shown to offer more precise measurements of the aortic root than echocardiography and thus reduces rates of paravalvular leak (PVL),7–10 an independent predictor of post-operative mortality from 30 days to 2 years.11–20
a, coronary arteries ostia and any bypass grafts should be assessed in all patients considered for TAVR.5 While 2D echocardiography provides valuable haemodynamic information and is readily available, a MSCT-based analysis of the aortic root and vascular access sites has become a sine qua non step in the pre-operative evaluation of patients for TAVR.6 Device sizing using multi-slice computer tomography (MSCT) has been shown to offer more precise measurements of the aortic root than echocardiography and thus reduces rates of paravalvular leak (PVL),7–10 an independent predictor of post-operative mortality from 30 days to 2 years.11–20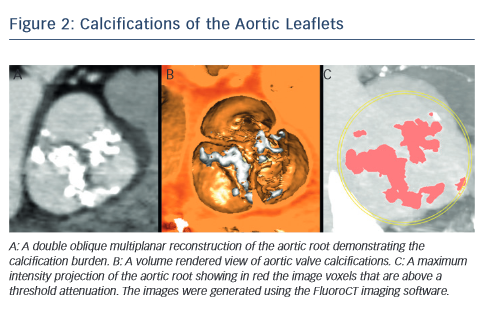
MSCT provides a precise method of evaluating aortic valve calcification (see Figure 2). Calcification is particularly important because it correlates with rates of post-intervention PVL9,21–24 It was recently demonstrated that rates of at least moderate PVL increase with left ventricular outflow tract (LVOT) calcification (OR 2.8, [95 % CI 1.2–7.0], P=0.022), and a leaflet calcium volume greater than 235 mm3 for a threshold of 850 HU (OR 2.8 [95 % CI 1.2–6.7], P=0.023).24
One of the most common complications of TAVR is a new left bundle branch (LBBB) or complete atrioventricular (AV) block requiring the implantation of a permanent pacemaker. The pathophysiology of these iatrogenic conduction abnormalities is the mechanical compression of the left bundle branch in the uppermost aspect of the muscular  interventricular septum by the TAVR implant (see Figure 3). An increased depth of implantation is the most frequently identified predictor of LBBB with both balloon- and self-expandable prostheses.25–30 In order to obtain an optimal implantation depth, the operator must minimise parallax inherent to
interventricular septum by the TAVR implant (see Figure 3). An increased depth of implantation is the most frequently identified predictor of LBBB with both balloon- and self-expandable prostheses.25–30 In order to obtain an optimal implantation depth, the operator must minimise parallax inherent to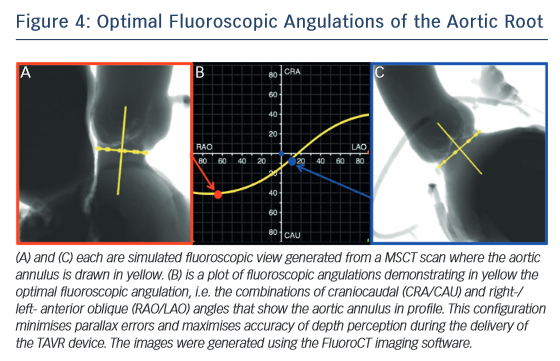 2D fluoroscopy used during the implantation.31 MSCT can be used to plan optimal C-arm angulations,32–41 i.e. angulations that present the axis of the aortic root parallel to the fluoroscopic detector (see Figure 4). In a recent study,41 such optimised angulations were associated with a significant decrease in implantation time (P=0.0001), radiation exposure (P=0.02), amount of contrast (P=0.001), and risk of acute kidney injury (P=0.03).
2D fluoroscopy used during the implantation.31 MSCT can be used to plan optimal C-arm angulations,32–41 i.e. angulations that present the axis of the aortic root parallel to the fluoroscopic detector (see Figure 4). In a recent study,41 such optimised angulations were associated with a significant decrease in implantation time (P=0.0001), radiation exposure (P=0.02), amount of contrast (P=0.001), and risk of acute kidney injury (P=0.03).
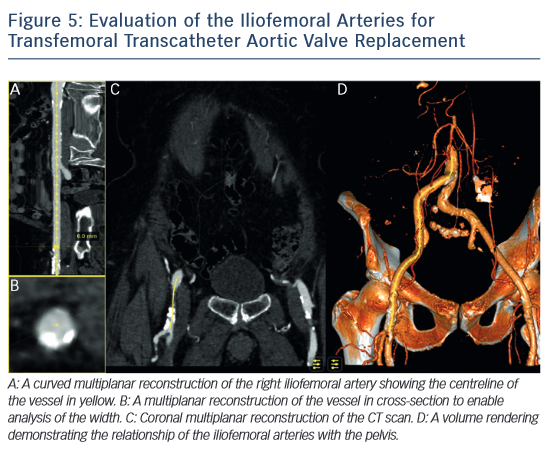
MSCT also plays a role in determining the suitability of access vessels in patients evaluated for TAVR. The majority of TAVR performed today is via the retrograde trans-femoral approach. This technique is the least invasive when compared with trans-apical, trans-aortic, trans-subclavian or trans-carotid TAVR.42–44 While less invasive, transfemoral TAVR can result in vessel dissection, stenosis, perforation, rupture, arterio-venous fistula, pseudoaneurysm, haematoma or nerve injury.45 The main risk factors of vascular injury secondary to the insertion of the catheter sheath include the vessel diameter, calcification, and tortuosity,46,47 (see Figure 5). Contrast-enhanced MSCT provides information about these three aspects within the same imaging study. The sheath-to-vessel diameter ≥1.05–1.12 is a predictor of vascular injury when measured with CT.47,48 The use MSCT correlates with a decrease in vascular complications.46 MSCT also yields a greater predictive value for vascular complications than plain angiography.48