The Latest Available Data on Polymer-Free Drug-eluting Stent Technology in Patients with Diabetes
Gennaro Sardella of Rome, Italy, introduced his presentation by outlining the causes of DES failure in patients with DM (see Figure 1). These include a prothrombotic state and resistance to antiplatelet drugs, for which prasugrel and ticagrelor may be indicated. Other causes include diffuse coronary artery disease (CAD), negative remodelling and smaller minimal lumen area (MLA) post procedure, as well as extensive CAD with more complex lesions and CAD progression in the non-stented segment. The latter may be managed by the use of a coronary artery bypass graft (CABG) and statins. Persistent polymer-related arterial inflammation may be overcome by the use of a polymer-free or absorbable polymer DES. However, these patients typically present with neointimal hyperplasia and impaired rapamycinanalogue inhibition, suggesting the need for a more efficacious DES – ideally a DES designed for patients with DM.
The clinical efficacy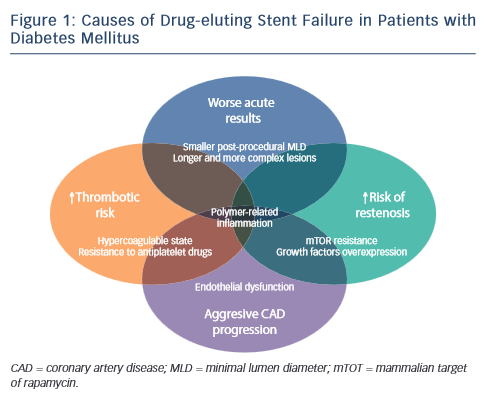 of the Cre8™ DES was evaluated in the International Randomized Comparison Between DES Limus Carbostent and Taxus Drug-Eluting Stents in the Treatment of De Novo Coronary Lesions (NEXT) clinical study, with a primary endpoint of 6-month angiographic in-stent late lumen loss (LLL). The study achieved its primary endpoint of non-inferiority compared with a permanent polymer DES Taxus (Liberté, Boston Scientific, US). However, the most striking finding of this study was that the LLL in the subgroup with diabetes was comparable to that in the general study population, a finding that had not been seen with other DES.1 At 3 years in the clinical follow-up a 37 % decrease in target lesion revascularisation (TLR) was reported in the Cre8™ group versus the Taxus group and a reduction of 68 % was seen in the population with diabetes. These findings led to further investigation of the Cre8™ DES in the population with diabetes.
of the Cre8™ DES was evaluated in the International Randomized Comparison Between DES Limus Carbostent and Taxus Drug-Eluting Stents in the Treatment of De Novo Coronary Lesions (NEXT) clinical study, with a primary endpoint of 6-month angiographic in-stent late lumen loss (LLL). The study achieved its primary endpoint of non-inferiority compared with a permanent polymer DES Taxus (Liberté, Boston Scientific, US). However, the most striking finding of this study was that the LLL in the subgroup with diabetes was comparable to that in the general study population, a finding that had not been seen with other DES.1 At 3 years in the clinical follow-up a 37 % decrease in target lesion revascularisation (TLR) was reported in the Cre8™ group versus the Taxus group and a reduction of 68 % was seen in the population with diabetes. These findings led to further investigation of the Cre8™ DES in the population with diabetes.
Another clinical study, ranDomizEd coMparisOn betweeN novel Cre8™ DES and BMS to assess neoinTimal coveRAge by OCT Evaluation (DEMONSTR8), assessed the Ratio of Uncovered to Total Stent Struts Per Cross Section (RUTTS) score, determined by optical coherence tomography (OCT) at 1 and 3 months for Cre8™ and a bare metal stent (BMS), and found that the Cre8™ DES at 3 months has comparable strut coverage to the BMS at 1 month, while preserving a greater efficacy in neointimal formation reduction.2
A propensity matched study (San Raffaele Scientific Institute, Milan, Italy) compared clinical outcomes of 187 real-world patients treated with the amphilimus polymer-free Cre8™ stent versus 150 patients receiving new-generation everolimus-eluting stents (EES) during the same period. It found superior clinical outcomes with Cre8™ compared with an EES in patients with diabetes.3
The MultIceNtric and RetrospectiVe REgiStry in ‘real world’ paTients with polymer-free drug elutInG stent Cre8™ (INVESTIG8) study has as its primary endpoint the incidence of clinical composite endpoint from baseline procedure to 12 months (cardiac death/ target vessel MI/clinically driven TLR). Secondary endpoints include the incidence of a clinical composite endpoint from the baseline procedure to 12 months (all deaths/all MI/any revascularisation); and the incidence of stent thrombosis from baseline procedure to 12 months, classified according to the Academic Research Consortium (ARC) definition. A recent subanalysis indicates that at 1 year, the composite endpoint was seen in 3.5 % of the population without diabetes and 5 % of those with diabetes. Clinically driven TLR was reported in 1.6 % of the population without diabetes versus 1.4 % of the population with diabetes. Freedom from events was seen in 97.8 % of the population without diabetes study and 95.6 % of those with diabetes. Definite and probable stent thrombosis occurred in only 0.6 % of the population without diabetes and 1.4 % of the population with diabetes.
In the Randomized Trial Comparing Reservoir-Based Polymer-Free Amphilimus‐Eluting Stents (AES) versus Everolimus-eluting Stents with Durable Polymer in Patients with Diabetes Mellitus (RESERVOIR) study, 112 patients with diabetes receiving glucose-lowering agents, were randomised to an amphilimus-eluting stent (AES) (Cre8™) stent or an EES with non-erodible polymer. The primary endpoint was neointimal volume obstruction at 9 months, evaluated by OCT. Secondary endpoints included strut coverage, angiographic in-stent late lumen loss and clinical endpoints such as target vessel revascularisation and probably/definite stent thrombosis.4,5 Results show that the Cre8™ was non-inferior to the EES and demonstrated non-significant superiority in the primary endpoint. In addition, the Cre8™ resulted in a lower LLL with a lower standard deviation, though without achieving statistical significance.
In conclusion, the ideal DES should combine safety with an increased DES efficacy. The Cre8™ DES has unique safety features (polymerfree structure, abluminal reservoir technology) and unique efficacy features (polymer-free structure and the amphilimus formulation), and is currently being tested in a large clinical programme. Data obtained so far suggest that the best application of Cre8™ seems to be the subset of patients with diabetes.
The publication of this article was supported by Alvimedica.