Percutaneous Coronary Intervention in a Patient with Dual Antiplatelet Therapy Constraints – Procedure and Clinical Outcome
Pieter Stella of Utrecht, The Netherlands, began by discussing PCI in the subsets of patients who have DAPT constraints: these include patients receiving oral anticoagulants (OACs), aged over 80 years, with previous bleeding events, with scheduled and urgent surgery and those non-compliant to medication.
The first factor associated with patients taking OACs is peri-procedural management during PCI. The choice of vascular access site is important, as is anticoagulant and antiplatelet management. The second factor is long-term management, where the risk of bleeding must be balanced against the risk of stent thrombosis. Factors that should be taken into consideration include bleeding risk, stroke risk (assessed by the presence of: congestive heart failure, hypertension, age ≥75 years, DM [CHADS2] score), whether or not the patient presents with ACS and the choice between BMS and DES. Age is also an important consideration; in octogenarians, the use of DES is associated with a lower cumulative death or MI rate (15.1 %) than with BMS (17.7 %).8
The duration of DAPT has been the subject of considerable recent debate. A recent meta-analysis of 10 randomised controlled trials (RCTs) and 31,666 patients investigated mortality with extended duration DAPT. Although treatment with DAPT beyond 1 year after DES implantation reduces MI and stent thrombosis, it was associated with increased mortality because of a 49 % increased risk of non-cardiovascular mortality, which is not offset by a reduction in cardiac mortality. It is worth mentioning that this could have been chance finding. In addition, prolonged DAPT has been associated with a 72 % risk of major bleeding.9 Therefore extended duration DAPT is not routinely recommended following PCI with stent implantation, but without DAPT, the risk of stent thrombosis increases.
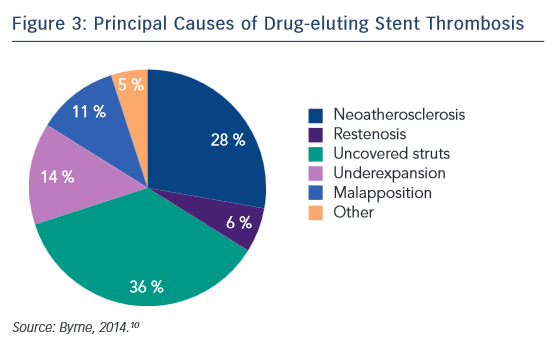
The principal causative factors of DES stent thrombosis are summarised in Figure 3.10 Of these, around 25 % are procedural-related issues, 36 % are DES safety-related issues and 34 % are DES efficacy-related issues. In a case-controlled study of 54 cases and 35 controls, the presence of uncovered stent struts was associated with stent thrombosis after DES: with a RUTTS score exceeding 30, stent thrombosis was seen in 21.6 % of cases versus none in cases with a RUTTS score less than 30.11 In terms of DES efficacy issues related to stent restenosis, a RCT showed that EES were comparable to sirolimus-eluting stents (SES) in terms of overall clinical efficacy and safety.12 Therefore, optimising the choice of DES can reduce stent thrombosis by approximately 70 %.
The Cre8™ DES features a polymer-free platform to reduce the risk of inflammation associated with durable polymers and the breakdown products of absorbable biopolymers (see Figure 2). A drug-delivery system, abluminal reservoir technology, utilises a formulation of sirolimus and a fatty acid, amphiphilic carrier. Sirolimus, an mTor inhibitor, is an immunosuppressant with antiproliferative and antimicrobial activity. In addition, it inhibits inflammatory cell activities and has high potency. The organic acid maintains sustained drug delivery; fatty acids are used to improve the transdermal delivery of numerous drugs.13 The fatty acid also enhances drug stability, as well as increasing the bioavailability of the drug; cardiac fatty acid uptake is doubled in diabetic mouse models.14 This results in an increased homogeneous drug distribution in diabetic cardiac cells. In addition, the bio inducer surface, a second-generation integral pure carbon coating, is designed to accelerate stent endothelialisation and strut coverage, thus reducing the risk of thrombosis. The Cre8™ DES is polymer-free, eliminating the disadvantages associated with durable polymers or their breakdown products.
To end the presentation, Professor Stella discussed his experience with implantation of the Cre8™ stent in the University Medical Centre, Utrecht. To date, 786 implantations have been performed, 372 with ACS and 414 with stable disease. All stable elective cases were treated with life-long aspirin and clopidogrel for 3 months; all ACS cases were treated with 12 months of DAPT. Outcomes to date have been excellent. In patients presenting with stable disease: at 6 months, stent thrombosis was seen in only 0.2 % of patients and TLR in 1.2 %. These findings have led to a new study design; an investigator-initiated randomised two-centre study, RECre8, which is currently enrolling, aims to recruit around 1,530 patients and is designed to compare the Resolute Integrity DES (Medtronic) versus Cre8™. Participants will be given 1 month DAPT in elective PCI and 12 months DAPT in ACS. Clinical follow up will be at 12 months and 3 years and will assess all MACE.
To end the presentation, Professor Stella presented a case of a 78-year-old man admitted for colon cancer, who suffered an ACS; an ECG showed inferolateral MI. Potential strategies included plain old balloon angioplasty (POBA), drug-eluting balloon (DEB) and implantation of a BMS or DES. The patient was treated with Cre8™ DES implantation and 4 weeks DAPT. At 4 weeks, OCT showed that all the struts were covered; therefore, the patient was referred for surgery and DAPT therapy was stopped. Six weeks later, an untreated residual stenosis was stented. The patient was doing well at clinical follow-up.
In conclusion, the Cre8™ polymer-free DES combines excellent efficacy with the safety associated with BMS, and offers the potential for reduced duration of DAPT. The ReCre8 study will further study the efficacy and safety of the Cre8™ stent with further reduced DAPT.
The publication of this article was supported by Alvimedica.