The AXXESS Stent
The Biosensors AXXESS Biolimus A9™ eluting coronary bifurcation stent system is a conically shaped self-expanding DES, constructed from a nickel-titanium alloy (nitinol). The stent is designed to dynamically adapt to the Y shape of the carina up to an angle of 70o, maintaining native geometry without metallic struts, and facilitating long-term SB access (Figure 2).
The stent has an abluminal polymer coating composed of polylactic acid, that is biodegradable into carbon dioxide and water within 6–9 months of deployment, and the semi-synthetic sirolimus analogue Biolimus A9™, which has antiproliferative effects and has been proven superior over first-generation sirolimus-eluting DES.9 The AXXESS™ stent is CE marked and has UK approval. It is currently available in 3.0 mm (expandable to 3.75 mm) and 3.5 mm (expandable to 4.25 mm) diameters, and in two lengths (11 and 14 mm). A 4.0 x 9 mm device has been developed and used in a limited number of cases in the UK, mainly in the left main stem (LMS), where a bifurcation angle of 120o can be spanned. However, this iteration of the device it is not currently available.
The AXXESS system is conventionally 7 French (7 Fr) compatible, although can be delivered through a specific 6 Fr guide catheter with an internal lumen diameter of 0.072” (e.g the Adroit guide, Cordis Miami, Florida, USA; but only with a single guide wire). It is delivered through a hydrophilic-coated rapid-exchange system comprising a delivery catheter, mounted stent and cover sheath (Figure 2). The stent is delivered following gradual withdrawal of this sheath, with the technique for implantation being described in detail below. One radiopaque gold marker exists at the proximal edge, with three at the distal edge arranged at equal intervals on the circumference to visually guide optimal placement (Figure 2). It is positioned spanning the carina into the ostium of both the MV and the SB.
Indications for Implantation
Any Medina classification of bifurcation can be treated using the AXXESS system. However, the angle between the MV and SB should not be greater than 70o and, ideally, the SB should be a minimum of 2.5 mm in diameter and have minimal calcification. In order to clearly define the bifurcation angle, two orthogonal radiographic projections are often required. As it elutes Biolimus A9, the standard contraindications for drug-eluting devices apply. There are no restrictions in location, with devices being used successfully throughout the coronary circulation, including the left main bifurcation;10,11 however, it cannot be overexpanded and careful vessel sizing (often requiring intravascular imaging) should be performed. It can be implanted for the full range of coronary interventional indications, including acute coronary syndromes and stable angina.12
AXXESS is particularly useful in the treatment of Medina class 1,0,0 lesions, as it is often the only device required in these cases. When treating all other Medina classes, additional distal overlapping DES in either MV or SB may be required.
Technique of Implanting an AXXESS™ Stent
Although the AXXESS device can be delivered through any 7 Fr-guiding system, when considering guide shape, support should be maximised to facilitate device delivery. Two separate guide wires (any moderate support 0.014” wire) should be advanced distally into the MV and SB. The use of dedicated stents, particularly self-expanding devices, require thorough lesion preparation, particularly in more complex calcified lesions (e.g. Medina 1,1,1). Consequently, noncompliant balloons, cutting balloons and adjunctive devices such as rotational atherectomy should be considered early to optimise final position, minimal luminal area and stent expansion. Passage of a ‘winged’ cutting balloon distal to the target lesion is an indicator that the AXXESS device can be delivered into the appropriate position. Predilatation is therefore strongly recommended before delivering the AXXESS stent, with vessel preparation of both MV and, in the majority of cases, into the ostium of the SB if disease is present.
The AXXESS™ stent is advanced onto the wire of the distal vessel with the sharpest bend to the proximal MV. This can be either branch, but in a series of over 100 implanted devices evaluated by our group it was positioned on the MV wire in 92 % of cases.10 The distal markers of the AXXESS stent are advanced beyond the carina in the distal vessel. The whole device is then gently retracted, alongside simultaneous gradual pullback of the actuator, slowly retracting the sheath. This allows progressive flaring of the three distal markers across the carina. Angiographically, this is visualised when one marker appears to be in either MV or SB, with the other two markers positioned in the other vessel, spanning the carina. While the cover sheath contains more than half the stent length (identified by the deployment marker on the delivery catheter), further adjustment of the stent position is possible. It is often helpful to confirm adequate carina spanning by gently advancing the device forward while the cover sheath remains in this position, as adjustments can still be made at this point. Thi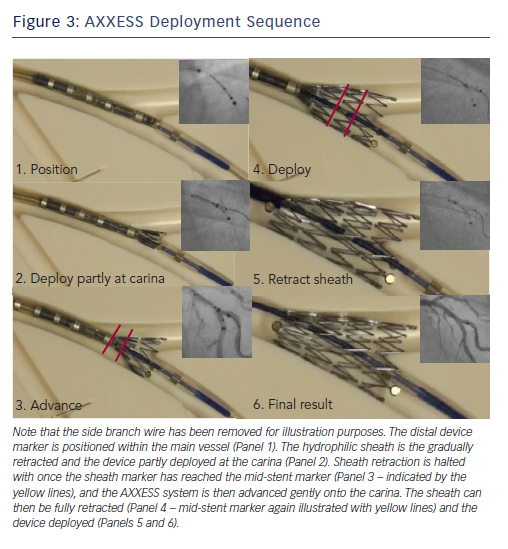
The final positioning manoeuvre occurs with gentle forward pressure applied with the device spanning the carina, and full retraction of the covered sheath using the actuator. Ideally, the device should be placed 2–3 mm distal to the carina to maximise distal vessel stent coverage (Figure 3).
Following deployment of the AXXESS stent, the jailed wire can then be withdrawn and reintroduced if needed, with easy access maintained into the other branch. Further stents can then be advanced if required and deployed in the distal MV or SB. If a distal stent is required, then the operator should aim for a minimal overlap of 1–2 mm between the AXXESS and any further stents. Similarly, significant proximal disease to the edge of the AXXESS stent in the MV can be treated with overlapping DES as appropriate. In the authors’ experience, Biomatrix devices have been used, conventionally, as they carry the same polymer and drug, although any DES could be used. As the AXXESS stent is self-expanding, post-dilatation is often not required. If no stent is placed in the SB distal to the AXXESS stent, often a final hugging balloon dilatation with the SB is performed. An appropriate non compliant (NC) balloon is placed within the distal marker of the AXXESS stent in the SB against an NC balloon in the MV. If both MV and SB were stented distal to the AXXESS stent, then standard final kissing balloon dilatation (FKBD) should be performed in the majority of cases.