Outcomes
There is a growing body of literature that supports the use of the AXXESS system in the treatment of coronary bifurcation lesions. The first-in-man AXXESS PLUS trial reported results at 6 months in 139 patients who underwent implantation across 13 centres, with low rates of TLR (7.5 %) and late-lumen loss (0.09 mm).13 There was a low rate of periprocedural complications (MACE rate 5% (n=7), non-Qwave MI 4.3% (n=6)), with a late-stent thrombosis rate of 2.1 % (three patients, two of who associated with premature cessation of antiplatelet therapy).
This trial has been followed with the prospective DIVERGE study, that assessed 302 patients across 14 centres in Europe, Australia and New Zealand.14 The cumulative MACE rate at 9 months was 7.7 % (0.7 % death, 3.3 % non Q-wave MI, 1.0 % Q-wave MI and 4.3 % TLR). This is comparable to the equivalent MV stent (Biomatrix) with the 9-month MACE rate in the Biolimus arm of the LEADERS trial being reported as 9.2 %.15 Importantly, 64 % of patients treated with AXXESS had additional DES (in both MV or SB) and all DES used were first-generation sirolimus-eluting devices. Stent thrombosis was observed in 1.0 % of patients (0.7 % subacute and 0.3 % late), the overwhelming majority of which occurred within the additional DES rather than the AXXESS device. The overall restenosis rate was 6.4 %. Both the AXXESS PLUS and the DIVERGE study have now reported 5 years results – pooled data represent a patient population of 432 patients. The overall MACE rate was reported at 21.3 % (6.5 % death, 8.6 % MI and 12.4 % ischaemia-driven TLR).16 This is comparable to the MACE rate of 22.8 % observed in the Biolimus arm of the LEADERS study at 5 years,9 and to the 5-year MACE rate of at 18.3 % in the MV-only arm in the NORDIC bifurcation trial.3
Importantly, among the DIVERGE cohort, 36 % of patients with true bifurcation lesions were treated using a single device only, or with a single additional stent in either MV or SB.17 This is an equivalent approach to a provisional technique, but maintaining SB access, and leaving the carina free from metallic scaffold. Indeed, the MACE rate in this sub-group was 18 %, again comparable to the provisional MV-only arm of the NORDIC trial.3 Importantly, this was not significantly different to the MV-plus-SB group using AXXESS (MACE rate of 23.3 %). Whereas, when a two-stent technique was employed within the NORDIC trial the overall MACE rate was 28.2 %, which was significantly different to the MV-only stent arm. The overall MACE rate for the MV arm of nordic bifurcation at 5 years was 18.3 %.
The DIVERGE14 and AXXESS PLUS trial13,15 both had stringent inclusion criteria, with the treated lesion dimensions restricted to just 10 mm extension into the MV proximal to the bifurcation, and 15 mm into either branch (MV and SB). They excluded lesions with angiographically apparent heavy calcification. It may be considered that this does not reflect ‘real-world’ practice, but observational data are available to support the use of AXXESS in more complex lesion subsets. In our data series from over 100 implants across four UK centres, the device has been successfully deployed in a variety of situations, including heavily calcified vessels that require rotablation, chronic total occlusions and in vessels that contain multiple bifurcations and where angles exceed 70o. The overall complication rate was low, with a single access site-related retroperitoneal bleed, and a single coronary perforation following post dilatation of the MV AXXESS stent, representing a 2 % complication rate,12 similar to that in other published series.16
The single randomised trial in the literature, the COBRA study,18 compares the use of the AXXESS system alongside Biomatrix s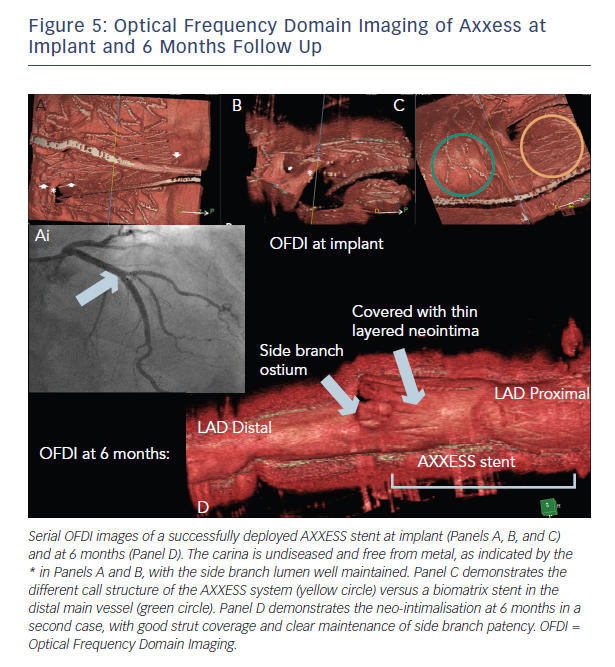
the DIVERGE study that demonstrated a 26 % increase in luminal volume at 6 months.19 This is likely to be a consequence of the self-expanding nature of the AXXESS device, maximising luminal gain from a PCI bifurcation procedure. The OCT findings at 6 months are illustrated in Figure 5.
There remains a lack of large-scale prospective randomised data evaluating the use of the AXXESS system. Despite this, cohort reports and the prospective data presented above have indicated that the system is safe, effective and with rates of adverse outcomes that are comparable to to that observed in large-scale randomised biolimus-eluting stent trials.