Special situations
Multiple leaks
In cases of multiple leaks, the authors recommend closing the major leak only at first, as if there is significant infection/hemolysis, the offending device can be identified. If multiple devices are placed, perhaps one is not the infectious source and therefore should not be removed. Th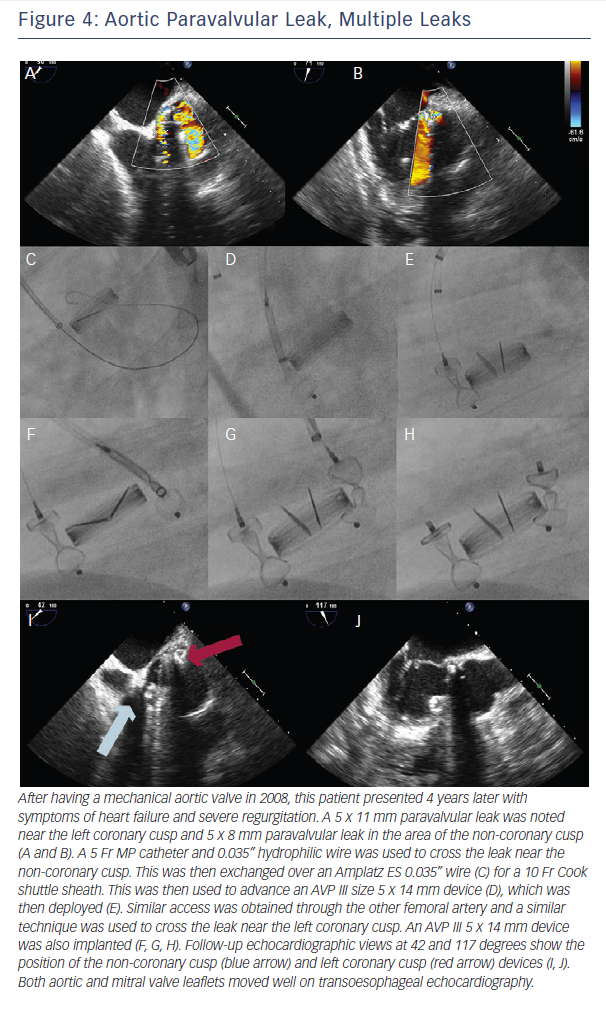 e authors place multiple devices or close multiple leaks if there is uncertain follow-up or with two equally sized large leaks.
e authors place multiple devices or close multiple leaks if there is uncertain follow-up or with two equally sized large leaks.
One approach for multiple device placement is the same-sheath approach: both devices go through the same sheath one after the other. Device one crosses the leak and is deployed. Next, the wire and delivery catheter are used to cross the leak again, and the second device is advanced and deployed. With this method, only one access is needed. However, the first device needs to be fully released before the second device can be advanced.
Another method is with new access. Contralateral femoral access and device advancement may be sufficient for aortic PVL (Figure 2). In the case of mitral PVL, this requires a transseptal approach and dilating the septal access point to accommodate a larger sheath. The larger sheath should be a sum of the sheaths required for the individual devices (if the two devices need two 6 Fr sheaths, the septum should be crossed with a 12 Fr sheath). Then two (or three wires for three devices) are used to cross the sheath, these wires are exchanged for stiff wires, and the prior large sheath is switched to the multiple delivery systems. The independent devices are then delivered (see Figures 4 and 5).
Preserving PVL access with device placement
When the wire crosses the leak only with great difficulty (e.g. tortuous anatomy and/or suboptimal transseptal catheter position), it is also possible to preserve PVL access during device placement with an 0.014” coronary wire (Figure 1). This allows the ability to preserve access – which is crucial if the device must be removed because it is not the correc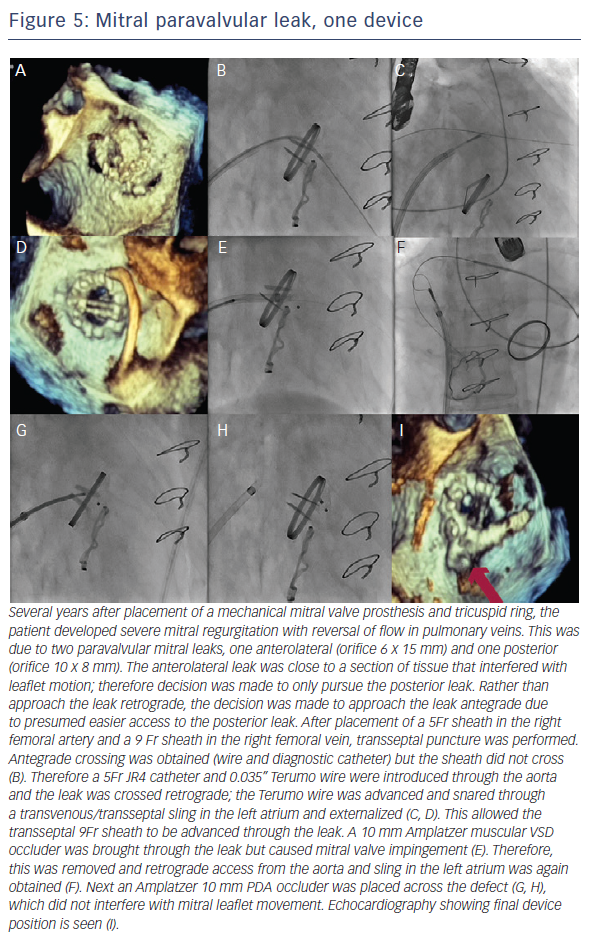 t size or causes leaflet compromise. However, this carries with it the risk that the wire will not be retrievable after the device is released.
t size or causes leaflet compromise. However, this carries with it the risk that the wire will not be retrievable after the device is released.
Complications
Complications will occur but must be avoided when possible. These include valve interference (3.5–5.0 %), stroke, endocarditis, post-procedural hemolysis, device erosion, emergent cardiac surgery (0.7–2.0 %) and death (1.4–2.0 %). One study showed major adverse events at 30 days (death, myocardial infarction, stroke, major bleeding and emergency surgery) at a rate of 8.7 %. Embolised devices from the aortic position are often large and go to the iliac bifurcation and can be removed percutaneously; those from the mitral position may be caught at the left ventricular outflow tract and may require surgery.
Devices that embolise from the aortic position may travel anywhere. Larger devices are less likely to locate cranially and are often found at the iliac bifurcation. The same holds true for devices that embolise from the mitral position, as most are small enough to pass through the left ventricular outflow tract and the aortic valve.
Post-procedural hemolysis is often due to shearing as blood flows through the now smaller orifice at a higher velocity. While this may worsen the clinical condition, this may also be well tolerated and resolves spontaneously after complete endothelialisation. This may take months.
Long-term survival
Technical success rate has reported as 77–86 %, and there has been 67–77 % clinical improvement. A study by Ruiz et al. reported long-term survival at 6, 12 and 18 months as 91.9, 89.2 and 86.5 %, respectively.8 Sorajja et al. found 1–2 year survival after PVL closure of 70–75 % with an estimated 3-year survival rate of 64.5 %.11