Antiplatelet Therapy
Current guidelines support the early administration of oral antiplatelet agents upstream of angiographic assessment and intervention.1 Aspirin is commonly given by the first medical contact and additional oral antiplatelet drugs are administered on arrival in hospital (see Figure 1).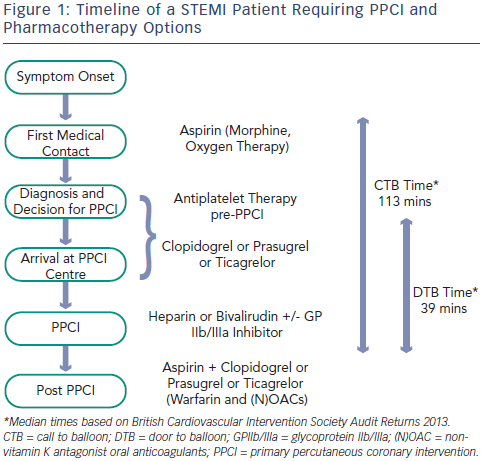
Aspirin
The efficacy of aspirin in acute ST-segment elevation myocardial infarction (STEMI) was first demonstrated in the Second International Study of Infarct Survival (ISIS-2).2 In ISIS-2, 17,187 patients were randomised within 24 hours of an acute STEMI to receive oral aspirin 160 mg/day for 30 days, intravenous streptokinase, both agents or neither drug. Compared with placebo, aspirin therapy resulted in a highly significant reduction in vascular mortality (23 % odds reduction [OR]), equivalent to streptokinase monotherapy (25 % OR). The combination of aspirin and streptokinase offered even greater benefit (42 % OR). Aspirin therapy was also associated with significant reductions in the incidence of non-fatal re-infarction (1.0 versus 2.0 %) and stroke (0.3 versus 0.6 %) with no increase in the risk of major bleeding or haemorrhagic stroke.
Aspirin has excellent bioavailability and this is enhanced by use of uncoated aspirin, administered chewed or crushed to establish a high blood level quickly (time to peak concentration [Tmax] 20–30 minutes).3 Interestingly, there is a significant geographic variation in the dosing of aspirin. In Europe, the recommended oral loading dose is 150–300 mg (or intravenous [i.v.] 80–150 mg) followed by 75–100 mg by mouth (p.o.) daily.1 US STEMI guidelines recommend 162–325 mg loading followed by 81–325 mg daily.4
Clopidogrel
Clopidogrel is a thienopyridine – a pro-drug requiring two cytochromep450 dependent steps to generate an active metabolite – which binds irreversibly to the P2Y12 adenosine diphosphate (ADP) receptor on platelets (see Figure 2). Genetic polymorphisms in the cytochrome P450 (CYP) enzymes can lead to lower levels of the active clopidogrel metabolite, diminished platelet inhibition and a higher rate of major adverse cardiovascular events (MACE), including stent thrombosis. Approximately 30 % of healthy subjects have been shown to be carriers of a reduced function CYP2C19 allele.5
Use of clopidogrel in STEMI patients has evolved from initial trials in acute coronary syndrome (ACS) patients undergoing percutaneous coronary intervention (PCI) (Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial [PCI-CURE])6 and patients with STEMI treated with fibrinolysis before PCI (PCI-clopidogrel as adjunctive reperfusion therapy trial [PCI-CLARITY]).7 In PCI-CURE, ACS patients undergoing PCI benefited from combined treatment with clopidogrel and aspirin, achieving a 31 % reduction in cardiovascular death and MI at 30 days. In PCI-CLARITY, clopidogrel pre-treatment in STEMI patients undergoing fibrinolysis led to a 46 % reduction in the 30-day rate of cardiovascular death, recurrent MI or stroke compared with placebo, without an increase in bleeding.
The recommended clopidogrel loading dose in STEMI patients is 600 mg. Results from the Intracoronary stenting and antithrombotic regimen: Choose between 3 high oral doses for immediate clopidogrel effect (ISAR-CHOICE) trial8 showed that in patients undergoing PCI, loading with 600 mg of clopidogrel (compared with 300 mg) resulted in higher plasma concentrations of the active metabolite and lower values for ADP-induced platelet aggregation 4 hours after drug administration. The clinical benefit of a 600 mg loading dose in STEMI patients undergoing PPCI was demonstrated in the Antiplatelet therapy for reduction of myocardial damage during angioplasty (ARMYDA)-6 MI,9 Clopidogrel and aspirin optimal dose usage to reduce recurrent events – seventh organisation to assess strategies in ischemic symptoms (CURRENT-OASIS) 710 and Harmonising outcomes with revascularisation and stents in acute myocardial infarction (HORIZONS-AMI)11 trials. In ARYMDA-6 MI, high dose loading reduced infarct size with improved cardiac function, and 30-day MACE rates. Similarly, in subgroup analyses of the CURRENT-OASIS 7 and HORIZONS-AMI trials, STEMI patients loaded with clopidogrel 600 mg, prior to PPCI, had a significant reduction in stent thrombosis and myocardial infarction, without any increase in bleeding events.
Prasugrel
Prasugrel is a third-generation thienopyridine, sharing the same active metabolite as clopidogrel (see Figure 2), and despite partial reliance on CYP2C19, achieves faster and more potent platelet inhibition (a 60 mg loading dose of prasugrel reaches maximal plasma concentration at 30 minutes in healthy volunteers).12 Prasugrel has a very low rate of nonresponders in comparison with clopidogrel.13
The clinical superiority of prasugrel over clopidogrel in ACS was demonstrated in the TRial to assess Improvement in Therapeutic Outcomes by optimising platelet inhibitioN – Thrombolysis in Myocardial Infarction-38 (TRITON–TIMI 38) study.14 Prasugrel, administered following angiography, reduced the composite primary endpoint (cardiovascular death, non-fatal MI or stroke) in patients undergoing PCI for STEMI or moderate-high risk ACS. In the pre-specified STEMI subgroup (3,534 patients), the risk redu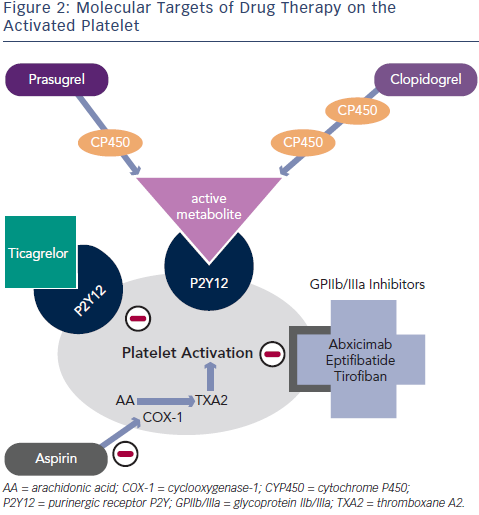 ction was 21 % (prasugrel 10 % versus clopidogrel 12.4 %) at 15 months, without a significant increase in non-coronary artery bypass graft (CABG)-related bleeding.15 The risk of stent thrombosis was also significantly lower.
ction was 21 % (prasugrel 10 % versus clopidogrel 12.4 %) at 15 months, without a significant increase in non-coronary artery bypass graft (CABG)-related bleeding.15 The risk of stent thrombosis was also significantly lower.
Prasugrel is contraindicated in patients with prior stroke/transient ischaemic attack (TIA), and is not recommended in patients aged ≥75 years or in patients with lower body weight (<60 kg), as there was no net clinical benefit in these subsets. A reduced maintenance dose of 5 mg could be considered in these patients.
Ticagrelor
A new chemical class called CycloPentylTriazoloPyrimidine is partly formed by Ticagrelor, which, in contrast to thienopyridines, causes reversible inhibition of the P2Y12 receptor and does not require hepatic metabolism for its activity (see Figure 2).16 Similar to prasugrel, ticagrelor provides more rapid, potent and consistent platelet inhibition over clopidogrel.
In the PLATelet inhibition and patient Outcomes (PLATO) trial,17 ticagrelor (compared with clopidogrel) reduced the composite primary endpoint (cardiovascular death, non-fatal MI or stroke) and also reduced cardiovascular mortality in STEMI and moderate–high risk ACS patients. In the STEMI subgroup, this primary endpoint was reduced from 10.8 % in the clopidogrel group to 9.4 % in the ticagrelor group (relative risk [RR] reduction of 13 %). In addition, overall mortality was reduced from 6 % to 4.9 % without a higher risk of major bleeding.
Dyspnoea is a frequently reported side effect of ticagrelor. In PLATO, 13.8 % of patients on ticagrelor reported dyspnoea compared with 7.8 % treated with clopidogrel.17 However few patients (0.9 %) discontinued the drug because of dyspnoea; importantly, there were no associated lung abnormalities and the mortality benefit persisted in this group.18 Contrary to the PLATO experience, a recent study of ticagrelor compliance in ACS patients demonstrated that dyspnoea was the commonest reason for drug discontinuation, occurring in 9.1 % of cases.19
The European Society of Cardiology (ESC) and American College of Cardiology Foundation/American Heart Association (ACCF/AHA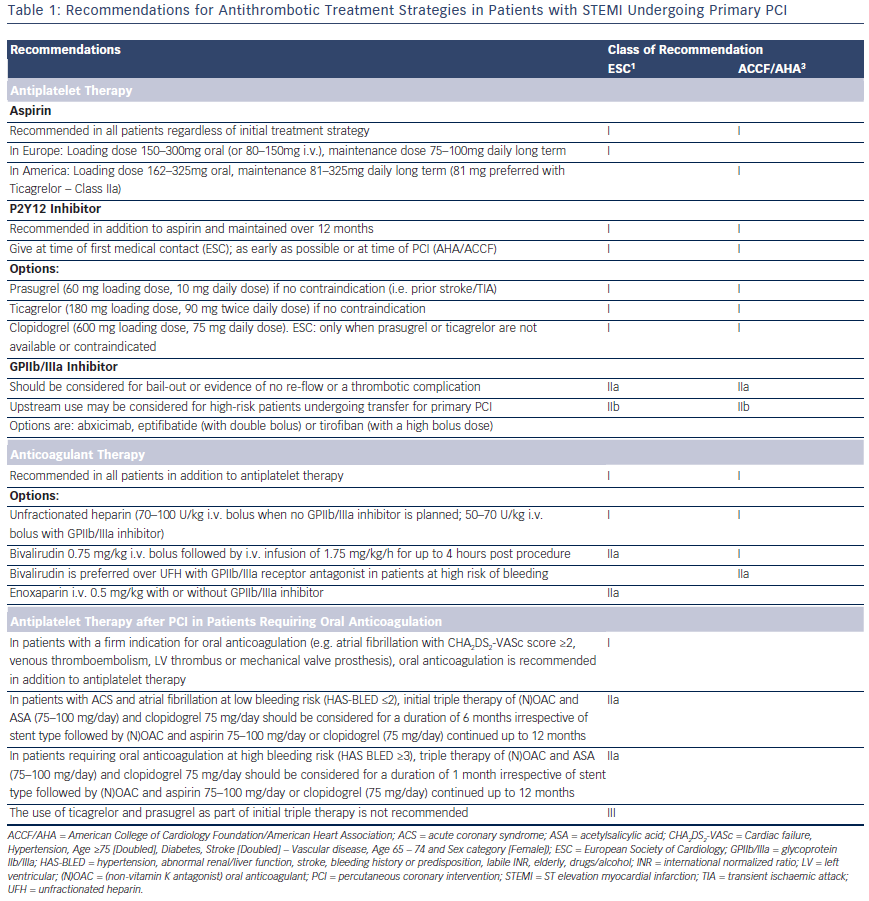 ) recommendations for antithrombotic strategies in patients with STEMI undergoing primary PCI are summarised in Table 1. Prasugrel, ticagrelor and clopidogrel (600 mg loading dose) are all class I, level B options in both guidelines, but the ESC expresses a clear preference for the newer antiplatelet agents, stating that clopidogrel should only be used when prasugrel or ticagrelor are either not available or contraindicated.
) recommendations for antithrombotic strategies in patients with STEMI undergoing primary PCI are summarised in Table 1. Prasugrel, ticagrelor and clopidogrel (600 mg loading dose) are all class I, level B options in both guidelines, but the ESC expresses a clear preference for the newer antiplatelet agents, stating that clopidogrel should only be used when prasugrel or ticagrelor are either not available or contraindicated.
Glycoprotein IIb/IIIa Inhibitors
Glycoprotein IIb/IIIa inhibitors (GPIs) provide rapid, potent platelet inhibition. Their use in PPCI has spanned the evolution of PCI and pharmacological therapies; consequently, it is challenging to relate the data to current practice with more potent oral antiplatelet therapies. Initial data supported the combined role of stenting and abciximab administration to minimise target vessel revascularisation,20 and preangiographic commencement of therapy appeared advantageous.21 However, in the dual antiplatelet therapy (DAPT) era, early use of abciximab resulted in an increased rate of bleeding.22 Subsequent analysis has demonstrated a continued benefit in early administration of GPIs in high-risk patients,23 particularly if presenting early or to a non-interventional centre.24 Contemporary trials provide conflicting results, the ONgoing Tirofiban in Myocardial infarction Evaluation 2 (ON-TIME 2) trial,25 utilising pre-hospital initiation of high bolus dose tirofiban, in addition to aspirin, heparin and high-dose clopidogrel, reduced MACE at 30 days with no significant increase in major bleeding. However, the HORIZONS-AMI26 trial demonstrated superiority of bivalirudin versus unfractionated heparin (UFH) and GPI in terms of a composite of major bleeding and MACE. Consequently, current guidelines1,4 suggest restricting GPI use for ‘bailout’ in the event of angiographic evidence of massive thrombus, slow-/no-reflow or a thrombotic complication.