Guidelines
Recently, the guidelines on the role of mechanical circulatory support in patients with STEMI complicated by CS have significantly changed. The European guidelines of 2010 recommended the use of IABP (class I/C) in patients with CS, whereas the guidelines of 2014 do not recommend the routine use of IABP anymore (class III/A) (see Table 1).46 The main reasons for changing the guidelines are the publication of a meta-analysis and a large randomised controlled trial evaluating the clinical benefit of the IABP, both showing no beneficial effect on 30-day mortality.27,29 The 2013 US guidelines recommend to assess the need for inotropic therapy, IABP or both on an individual basis, as observational data are conflicting. The superior haemodynamic support of LV assist devices is mentioned, but with the annotation that the experience is limited. In these guidelines, IABP has a class IIa recommendation in patients who do not quickly stabilize with pharmacological therapy. Alternative LV assist devices may be considered in patients with refractory CS (class IIb). An overview of recommendations of using mech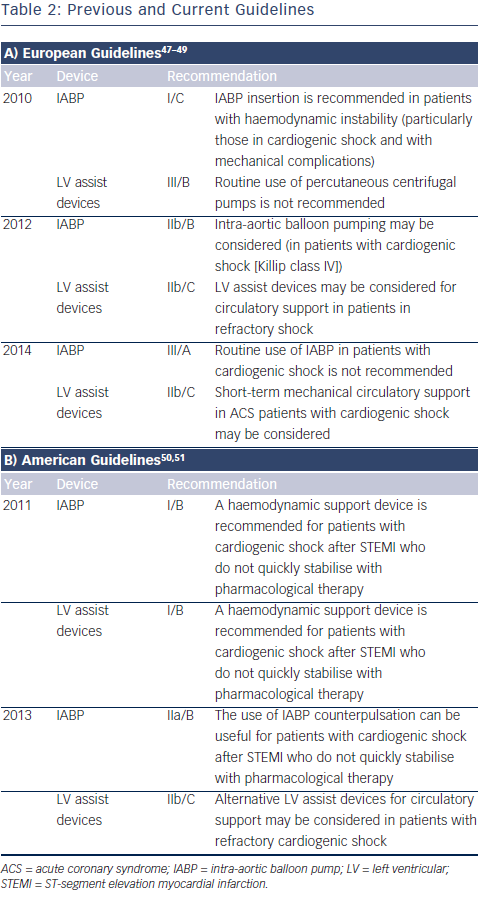 anical assist devices in CS is shown in Table 2.47–51
anical assist devices in CS is shown in Table 2.47–51
Future Perspectives
The experience of usage of mechanical assist devices is expanding rapidly. The indications include not only acute CS patients and highrisk PCI, but also as a bridge-to-transplant in advanced heart failure patients. In the forthcoming years, the development and the usage of percutaneous assist devices will increase and haemodynamic support will be used more frequently as an additional treatment in several patient groups. As recent studies were not able to show a survival benefit of the frequently used IABP in CS after myocardial infarction, the experience with other assist devices will expand in this setting. Other assist devices have not yet shown their benefit on survival, and are usually not available outside expert centres. As many attending cardiologists often feel the need to do more than just initiate pharmaceutical inotropic support, IABP will probably continue to be used until alternatives are widely available. However, it is important that other assist devices show clinical effectiveness before they become common clinical practice.
For usage of mechanical assist devices during high-risk PCI, there are two important questions that need to be answered. First, the question rises in terms of the role of mechanical circulatory support during these procedures. Is there a clinical benefit and, if so, in which patients? Second, which support device is the most optimal in this setting? The complications should not outweigh the possible clinical benefit of these invasive devices.
Future developments on mechanical assist devices need to focus on minimising insertion-point-related complications like limb-ischaemia and severe bleeding by reducing the device size while maintaining sufficient haemodynamic support. Also thrombo-embolic complications should be reduced and the associated morbidity needs to be minimised. New mechanical support devices, such as the pulsatile ECMO (Pulsecath) and the Reitan catheter pump (Cardiobridge), are being developed. The usage of percutaneous RV assist devices in case of RV failure is in development but only little experience is available.
Choosing the appropriate mechanical support device may depend on the degree of support needed. In consequence, subgroups of patients regarding the severity of CS have to be defined to allow a better discrimination between patient groups and devices to detect beneficial or harmful effects on outcome in different subgroups.