Transcatheter aortic valve implantation (TAVI) has become a highly effective and beneficial treatment option for patients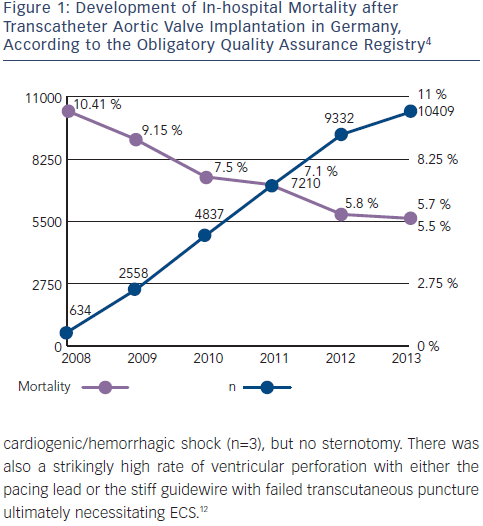 with severe symptomatic aortic valve stenosis. The randomized Placement of AoRtic TraNscathetER Valves (PARTNER) trials have shown that (1) TAVI using the balloon-expandable Edwards Sapien valve implanted via the transfemoral (TF) or transapical (TA) route is superior with respect to outcomes compared with standard therapy in inoperable patients and that (2) TAVI is at least non-inferior to surgical aortic valve replacement (AVR) in high-risk patients.1,2 More recently, the randomized US pivotal CoreValve high-risk study has reported that TAVI exclusively via the transvascular (83 % TF, 17 % trans-subclavian) approach using the selfexpandable Medtronic/CoreValve prosthesis is in fact superior to open surgery in such high-risk patients.3
with severe symptomatic aortic valve stenosis. The randomized Placement of AoRtic TraNscathetER Valves (PARTNER) trials have shown that (1) TAVI using the balloon-expandable Edwards Sapien valve implanted via the transfemoral (TF) or transapical (TA) route is superior with respect to outcomes compared with standard therapy in inoperable patients and that (2) TAVI is at least non-inferior to surgical aortic valve replacement (AVR) in high-risk patients.1,2 More recently, the randomized US pivotal CoreValve high-risk study has reported that TAVI exclusively via the transvascular (83 % TF, 17 % trans-subclavian) approach using the selfexpandable Medtronic/CoreValve prosthesis is in fact superior to open surgery in such high-risk patients.3
With rapidly increasing experience, TAVI globally has become a safe clinical routine procedure. In Germany, 30-day mortality rates have almost been halved since 2008 (see Figure 1), according to data from the German obligatory quality assurance registry (AQUA registry).4 Although the risk for severe complications such as annular rupture, ventricular perforation, or aortic injury during TAVI is low, ranging between 0.2 % and 1.0 % (see Table 1),5 some of these complications may ultimately require emergency cardiac surgery (ECS).