Anatomical Assessment and Intervention Guidance
Various studies have demonstrated advantages of IVUS guided interventions when compared to angiography.1–12 An analysis 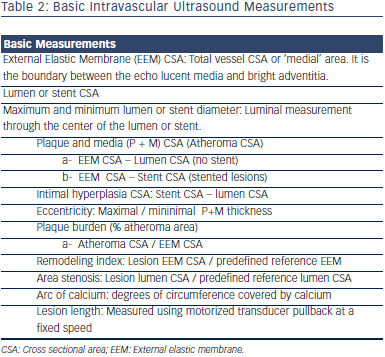 of the Strategy and intracoronary ultrasound-guided PTCA and stenting (SIPS) trial4 noted a 60.9 % probability that IVUS was less expensive and more effective when compared to angiographic guided interventions. Similarly, Gaster et al. demonstrated decreased cost with an IVUS guided intervention strategy.5 Intravascular ultrasound confers improved accuracy for lesion quantification (e.g. lumen, vessel wall and plaque diameter, area, length, shape and volume), and morphology assessment (e.g. aneurysms, bifurcations, ostial lesions, fibrosis and calcification patterns, filling defects, thrombus, intimal disruption, dissection and ulceration). Additionally IVUS shows the calcium distribution pattern within the vessel wall.13 IVUS is also more accurate than angiography for assessment of eccentric lesions.14 IVUS can aid in the identification of the culprit lesion in unclear cases and clarify the mechanism of Stent thrombosis (ST) or in-stent restenosis (ISR).15,16 Distal embolisation or peri-procedural myocardial infarction (MI) during interventions may be predicted with the IVUS presence of ruptured plaque and large plaque burden in acute coronary syndrome (ACS) and non-ACS patients.17 Greater attenuation angle (>180 degrees), and attenuation length >5 mm seem to be independent predictors for microvascular obstruction in ST segment elevation myocardial infarction (STEMI) patients undergoing primary PCI.18 Pre-emptive use of filter wires in these patient subgroups may prove beneficial. Table 2 and Figure 2 show common IVUS measurements and morphologic findings.
of the Strategy and intracoronary ultrasound-guided PTCA and stenting (SIPS) trial4 noted a 60.9 % probability that IVUS was less expensive and more effective when compared to angiographic guided interventions. Similarly, Gaster et al. demonstrated decreased cost with an IVUS guided intervention strategy.5 Intravascular ultrasound confers improved accuracy for lesion quantification (e.g. lumen, vessel wall and plaque diameter, area, length, shape and volume), and morphology assessment (e.g. aneurysms, bifurcations, ostial lesions, fibrosis and calcification patterns, filling defects, thrombus, intimal disruption, dissection and ulceration). Additionally IVUS shows the calcium distribution pattern within the vessel wall.13 IVUS is also more accurate than angiography for assessment of eccentric lesions.14 IVUS can aid in the identification of the culprit lesion in unclear cases and clarify the mechanism of Stent thrombosis (ST) or in-stent restenosis (ISR).15,16 Distal embolisation or peri-procedural myocardial infarction (MI) during interventions may be predicted with the IVUS presence of ruptured plaque and large plaque burden in acute coronary syndrome (ACS) and non-ACS patients.17 Greater attenuation angle (>180 degrees), and attenuation length >5 mm seem to be independent predictors for microvascular obstruction in ST segment elevation myocardial infarction (STEMI) patients undergoing primary PCI.18 Pre-emptive use of filter wires in these patient subgroups may prove beneficial. Table 2 and Figure 2 show common IVUS measurements and morphologic findings.
Mechanised pullb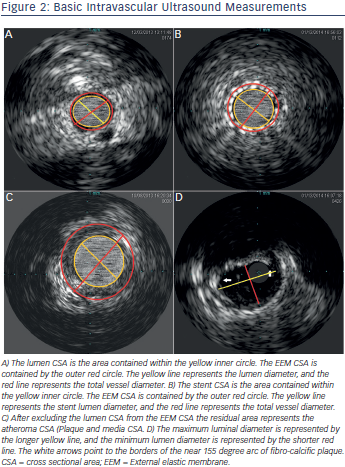 ack at a stable speed allows for length calculation19 but limits dynamic interactions by the operator that are available during manual pull-back. Knowledge of the distance between IVUS catheter markers may alternatively be used to estimate length when combined with fluoroscopy (see Figures 3 and 4). Eagle Eye® Platinum Rx Digital IVUS Catheters, (Volcano Corporation, Rancho Cordova, CA) have 14 mm from transducer to its first radiopaque marker, and a total of three markers each 10 mm apart. A short tip version of the catheter is available (Eagle Eye® Platinum ST Rx Digital IVUS Catheters, Volcano Corporation, Rancho C
ack at a stable speed allows for length calculation19 but limits dynamic interactions by the operator that are available during manual pull-back. Knowledge of the distance between IVUS catheter markers may alternatively be used to estimate length when combined with fluoroscopy (see Figures 3 and 4). Eagle Eye® Platinum Rx Digital IVUS Catheters, (Volcano Corporation, Rancho Cordova, CA) have 14 mm from transducer to its first radiopaque marker, and a total of three markers each 10 mm apart. A short tip version of the catheter is available (Eagle Eye® Platinum ST Rx Digital IVUS Catheters, Volcano Corporation, Rancho C ordova, CA). Atlantis® SR Pro Coronary Imaging Catheter (Boston Scientific Corporation, Natick, MA) has a 2.1 cm distance from marker band to its transducer. The OptiCross™ Coronary Imaging Catheter, (Boston Scientific Corporation, Natick, MA) has a 1 cm telescope marker that allows to calculate the manual pullback distance.
ordova, CA). Atlantis® SR Pro Coronary Imaging Catheter (Boston Scientific Corporation, Natick, MA) has a 2.1 cm distance from marker band to its transducer. The OptiCross™ Coronary Imaging Catheter, (Boston Scientific Corporation, Natick, MA) has a 1 cm telescope marker that allows to calculate the manual pullback distance.
Vessel areas of interest, (e.g. bifurcations, ostium, beginning and end of diseased vessels) can be accurately localised using the transducer marker adjacent to the imaging window. This is done by positioning the transducer at the area of interest followed by cine acquisition of the transducer marker. This marker position is compared to other adjacent fluoroscopic landmarks for future orientation. This technique allows for optimal geographic landing of interventional equipment. Gentle flushing is advised when injecting contrast during imaging for marker position evaluation, as brisk flushing may result in forward transducer translation producing a catheter motion artifact. Similarly, careful attention to catheter position is important to detect motion during cardiac or breathing cycle variation. IVUS guided PCI of native aorto-ostial, or ostial left anterior descending, left circumflex, or ramus intermedius lesions has been associated with lower rates of the composite of cardiovascular death, myocardial infarction (MI) or target lesion revascularisation (TLR) (hazard ratio [HR] 0.54, 95 % CI 0.29-0.99; p = 0.04), composite MI or TLR (HR 0.39, 95 % CI 0.18-0.83; p = 0.01) and MI (HR 0.31, 95 % CI 0.11–0.85; p = 0.02), as well as a trend towards a lower TLR rate (HR 0.42, 95 % CI 0.17-1.02; p = 0.06) compared with no IVUS.20
Although not available in the US, Boston Scientific offers iMap tissue characterisation software which uses radiofrequency sign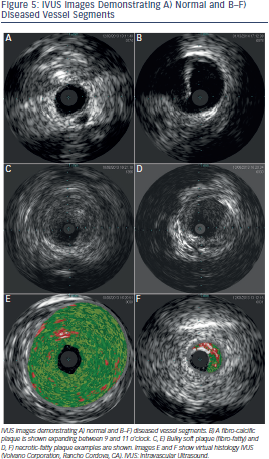 al spectrum pattern recognition to characteris
al spectrum pattern recognition to characteris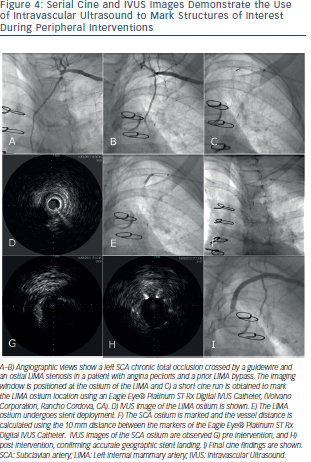 e tissue within the plaque.21 Boston Scientific also offers volumetric analysis software. Volcano offers virtual histology software (VH® IVUS) that identifies signal intensity and frequency variations and assigns different colours to each specified category with the goal of tissue composition characterisation, fibrous, fibro-fatty, necrotic-lipid and calcific, (see Figure 5).22 Volcano also offers ChromaFlo®; a colour flow function that highlights changes between serial frames and may assist in the identification of intraluminal filling defects (e.g. thrombus, unopposed stent struts, vessel dissection). Superficial echo attenuated plaques have been associated with advanced necrotic core containing fibroatheromas which are considered a high risk plaque pattern.23 However, secondary non-culprit ruptures frequently seen in ACS patients with this plaque phenotype do not seem to be associated with adverse outcomes on patients treated with optimal medical therapy.24 The clinical application of non-culprit plaque characterisation is therefore unclear at this time.
e tissue within the plaque.21 Boston Scientific also offers volumetric analysis software. Volcano offers virtual histology software (VH® IVUS) that identifies signal intensity and frequency variations and assigns different colours to each specified category with the goal of tissue composition characterisation, fibrous, fibro-fatty, necrotic-lipid and calcific, (see Figure 5).22 Volcano also offers ChromaFlo®; a colour flow function that highlights changes between serial frames and may assist in the identification of intraluminal filling defects (e.g. thrombus, unopposed stent struts, vessel dissection). Superficial echo attenuated plaques have been associated with advanced necrotic core containing fibroatheromas which are considered a high risk plaque pattern.23 However, secondary non-culprit ruptures frequently seen in ACS patients with this plaque phenotype do not seem to be associated with adverse outcomes on patients treated with optimal medical therapy.24 The clinical application of non-culprit plaque characterisation is therefore unclear at this time.
IVUS may clarify difficult scenarios that are uncertain by angiography, (e.g. left main coronary artery (LMCA disease), significance of inflow or outflow disease, bifurcation classification including evaluation for side branch disease), and aid in the selection of an optimal technical approach to interventions. Determining morphological characteristics associated with decreased vessel compliance, (e.g. extended arc of calcium or significant fibrosis) may assist the decision to use preemptive atherectomy.25