Optimum Antiplatelet Therapy in Non-ST Elevation Acute Coronary Syndrome (NSTE-ACS)
Patients with NSTE-ACS are at high risk for major adverse events and do also benefit from the new antiplatelet agents. However, NSTEACS patients tend to be older and have more co-morbidities such as chronic renal failure, and therefore there is an increased risk for bleeding complications.
In the NSTE-ACS cohort of the TRITON-TIMI 38 trial prasugrel significantly reduced the primary endpoint of cardiovascular death, non-fatal MI, or non-fatal stroke (HR=0.82, P=0.002), but was accompanied by an increase in the rate of major bleeding complications (HR 1.40, P=0.02).23 However, in patients who met the criteria for prasugrel use recommended by the European Medicines Agency (EMEA), thus excluding patients from the analysis with prior TIA/stroke, with weight <60 kg or age ≥75 years, prasugrel was superior with regards to ischaemic events without significant differences in non-CABG major bleedings. Most recently, the Comparison of prasugrel at the time of PCI or as pretreatment at the time of diagnosis in patients with non- ST elevation myocardial infarction (NSTEMI) (ACCOAST) trial has been published.24 Over 4,000 NSTEMI patients who were scheduled to undergo coronary catheterisation within 48 hours were included in this study. Patients were randomised to prasugrel (30 mg loading dose) or placebo before coronary angiography. When PCI was indicated, an additional loading dose of 30 mg prasugrel was given in the pretreatment group and a 60 mg loading dose was given in the control group. Pretreatment with prasugrel before angiography did not reduce ischaemic events (10.0 versus 9.8 %, HR=1.02; P=0.81), but was associated with more TIMI major bleeding (2.6 vs 1.4 %, HR=1.90, P=0.006).
The Targeted platelet inhibition to clarify the optimal Strategy to medically managed acute coronary syndromes (TRILOGY-ACS) is also a recent study which examined the effect of prasugrel in patients not undergoing revascularisation.25 Patients were randomised in the study only after a decision for medical management without revascularisation was made. At a median follow-up of 17 months Prasugrel did not significantly reduce the primary endpoint in comparison to clopidogrel (13.9 vs 16.0 %, HR=0.91; P=0.21). The results of TRILOGY-ACS do not support extending the indication for prasugrel to medically managed patients with NSTE-ACS.
There is also a post-hoc secondary analysis of the NSTE-ACS PLATO cohort. Consistent with the results in the overall population ticagrelor significantly reduced the primary endpoint (10.0 vs 12.3 %; HR=0.83; P=0.0013), cardiovascular (3.7 vs 4.9 %; HR=0.77; P=0.0.007) and total mortality (4.3 versus 5.8 %; HR=0.76; P=0.002) in patient with and without revascularisation.24 Major bleeding rate did not differ between the two treatment groups (13.4 vs 12.6 %; HR=1.07; P=0.26), but ticagrelor was associated with an increase in non-CABG major bleeding (4.8 vs 3.8 %; HR=1.28; P=0.0139).
There is no randomised comparison of prasugrel vs ticagrelor. However, Biondi-Zoccai et al. conducted an adjusteded indirect meta-analysis comparing both agents in patients with ACS.27 Head-to-head comparison suggested similar efficacy of prasugrel and ticagrelor, but p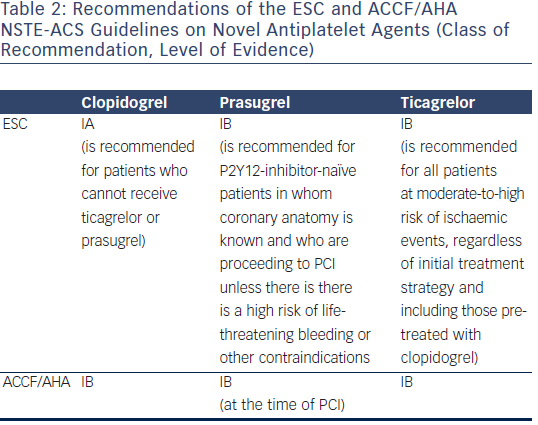 rasugrel appeared to be more benefical with regard to the occurrence of stent thrombosis, while increasing the risk of bleeding complications.
rasugrel appeared to be more benefical with regard to the occurrence of stent thrombosis, while increasing the risk of bleeding complications.
There are two important advantages of ticagrelor over prasugrel in the setting of NSTE-ACS. First, according to the ESC guidelines a P2Y12 inhibitor should be added to ASA as soon as possible,26 and ticagrelor can be given without knowledge of the coronary status. In the ACCOAST trial administration of prasugrel before coronary angiography was not associated with a net clinical benefit. Secondly, ticagrelor also improves prognosis in medically managed patients.26
The current NSTE-ACS guidelines of the ESC prefer ticagrelor and prasugrel over clopidogrel28 (see Table 2). Clopidogrel is only recommended for patients who cannot receive ticagrelor or prasugrel (IA recommendation). In contrast to the European guidelines the ACCF/AHA NSTE-ACS guidelines do not endorse prasugrel or ticagrelor over clopidogrel.29
There is only little data on the use of the novel antiplatelet agents in daily clinical practice. Randomised clinical trials usually enroll selected patient populations that may not be representative for patients seen in everyday practice. In an analysis of the Greek Antiplatelet registry (GRAPE) registry 24.1 % and 37.2 % of the patients with ACS undergoing PCI received prasugrel and ticagrelor, respectively.30 However, solid data is lacking, whether the novel antiplatelet agents also improve prognosis in an all-comer population.
In summary, ticagrelor may be the best option for most patients with NSTE-ACS, in particular in patients managed conservatively. Prasugrel may be best suited for younger patients with scheduled PCI and large areas of myocardium at risk or diabetes mellitus who have a low risk of bleeding. Again, clopidogrel should rather be used in patients with a contraindication for prasugrel and ticagrelor or concomitant chronic anticoagulation therapy.