Section A
Coronary Chronic Total Occlusions – Prevalence and Pathophysiology
A chronic total occlusion (CTO) is defined as a completely occluded coronary artery with no antegrade flow (thrombolysis in myocardial infarction [TIMI] 0 flow) for at least three months.1 CTOs are present in 15–30 % of patients undergoing coronary angiography.2–5 In a Canadian prospective registry of 14,439 patients undergoing coronary angiography a CTO was present in 18.4 % of all patients with significant coronary artery disease (CAD).2 Approximately 1/3–1/26,7 of patients undergoing CTO percutaneous coronary intervention (PCI) have had a prior acute myocardial infarction (MI). This suggests acute onset of the occlusion, whereas in the remaining patients gradual development of CTO from high-grade lesions likely occurred.
The basic histopathologic feature of a CTO is a proximal cap of the occlusion. This is often fibrotic or calcified and may provide considerable resistance to wire advancement during CTO PCI. Distal to the proximal cap and along the occlusion length follows a segment of loose fibrous tissue or organised thrombus, with various extent of calcification.8,9 In several of these lesions, residual channels may be observed that are not visible under angiography. In addition, microchannels may appear during the CTO’s consolidation process, however these are mostly located in the adventitia with extremely tortuous courses and do not generally traverse the entire occluded segment.10 A recent autopsy study of 95 CTO lesions from 82 patients reported frequent negative remodelling of the CTO body (more frequent with longer duration of the occlusion), very rare presence of microchannels and more frequent tapering of the distal cap as compared with the proximal cap (79 % vs. 50 %, P<0.0001).11
Collaterals are interarterial connections that provide blood flow to a vascular territory whose original supply vessel is obstructed. Thus, the integrity of the myocardium supplied by the obstructed vessel may be preserved, or to a certain degree impaired, but would not become necrotic. Collaterals develop through arteriogenesis, i.e. the recruitment of preformed and preexisting interarterial connections, which is driven mainly by shear forces along the pressure gradient that develops when the native vessel is occluded.12 The functional assessment of collaterals revealed that, in patients 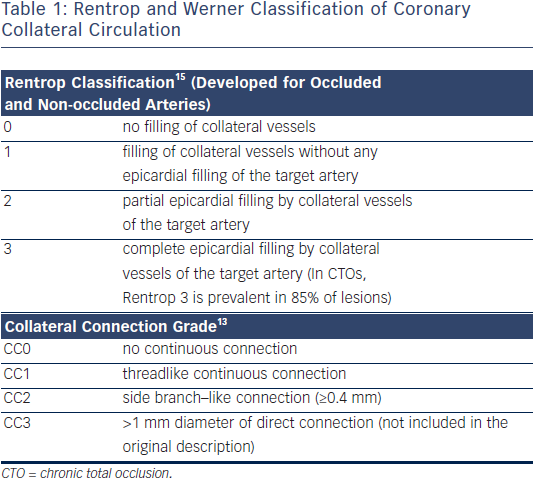 without well-developed preexisting collateral connections, collaterals require between 2–12 weeks to fully develop their functional capacity.13 The collateral supply provides a perfusion pressure in the range of 30–40 mm Hg at the occluded territory, a pressure that leads to the functional reduction of distal vessel size, which then leads to the underestimation of the vessel dimensions during a recanalization procedure.14
without well-developed preexisting collateral connections, collaterals require between 2–12 weeks to fully develop their functional capacity.13 The collateral supply provides a perfusion pressure in the range of 30–40 mm Hg at the occluded territory, a pressure that leads to the functional reduction of distal vessel size, which then leads to the underestimation of the vessel dimensions during a recanalization procedure.14
The most widely used angiographic grading system for collaterals (described by Rentrop et al. in 1985) does not actually rate the collaterals themselves but rather their effect in filling the occluded arterial segment15. Recently, a grading of collateral connections was introduced specifically for CTOs, which can help plan the retrograde approach13,16 (see Table 1). Collateral function can develop to a similar functional level in patients with prior MI and large akinetic territories, as in patients with preserved regional function, i.e. viability is not required for collateral development.17
The direct assessment of collateral function shows that the functional competence of collaterals in CTOs is limited, even in patients without a prior Q-wave MI. During a standard stress protocol with systemic infusion of adenosine, the coronary flow velocity and pressure changes distal to an occlusion (after CTO crossing but before stent implantation) were well below the cut-off values for assessing the functional reserve in non-occlusive coronary obstructions, i.e. a fractional flow reserve (FFR) above 0.75. Therefore, even well-developed collaterals do not prevent ischaemia during exercise.18–20
Collaterals will regress once the native artery that was replaced by the collaterals is revascularised.21 This process starts immediately after re-establishing antegrade flow with immediate loss of collateral conductance and lasts for many months after the revascularisation procedure.
The authors would like to thank Ms Sheila Agyeman for her invaluable effort in coordinating the manuscript creation process.