Transcatheter aortic valve implantation (TAVI) is an established alternative to surgical valve replacement in the management of calcified severe aortic stenosis in those with co-morbidities or adverse features (advanced age, impaired left ventricular function), or in those where open surgery may be associated with unfavourable technical features, such as previous sternotomy with a patent internal mammary graft, porcelain aorta or previous thoracic radiation, rendering the operative field hostile.1–3 A large body of experience and evidence exists predominantly for two commonly used TAVI devices; namely the balloon-expandable Edwards Sapien Valve (ESV) (Edwards Lifesciences Ltd, Irvine, California, US) and the self-expanding Medtronic CoreValve (MCV) (Medtronic Inc, Minneapolis, Minnesota, US). Although the fundamental principle behind the two valves is similar, both involving a stent with three bioprosthetic cusps (leaflets) deployed within a calcified native aortic valve, the specific design features and potential indications are different. In this article we explore in detail the respective design features, related indications for each valve type as well as their known outcomes.
General Indications for Aortic Valve Implantation
Normal aortic valve area is between 3 and 4 cm2 and a transvalvular gradient develops when the va lve area decreases by half. Patients with a normal left ventricle are generally asymptomatic unless the valve area is <1 cm2, with an accompanying jet velocity of approximately 4 m/s and a mean gradient >40 mmHg (see Table 1).4 Symptoms may occur at lower gradients and with less obstruction in some patients, such as those with poor left ventricular function (low flow aortic stenosis).
lve area decreases by half. Patients with a normal left ventricle are generally asymptomatic unless the valve area is <1 cm2, with an accompanying jet velocity of approximately 4 m/s and a mean gradient >40 mmHg (see Table 1).4 Symptoms may occur at lower gradients and with less obstruction in some patients, such as those with poor left ventricular function (low flow aortic stenosis).
Besides native aortic valve disease, it is important to consider that surgically implanted bioprosthetic valves also undergo structural deterioration resulting in calcification, wear and tear, thrombosis, pannus formation and docarditis.5 This deterioration can manifest as aortic stenosis, regurgitation or mixed disease. Although in a bid to avoid redo surgery, valve-in-valve TAVI is emerging as a treatment option in this group of patients, it is beyond the scope of this article.1
Patient Selection Criteria and Contraindications for Transcatheter Aortic Valve Implantation
The patient selection criteria for TAVI are essentially the same as for surgical valve replacement. The main reason for opting for TAVI is the presence of co-morbidities placing the patient in either the inoperable or the high-risk category, making the option of open surgery less favourable. Current recommended patient selection criteria for TAVI from the European Association of Cardio-Thoracic Surgery (EACTS) include one or more of the following:6
Numerous exclusion criteria are present, although none are absolute. The presence of a bicuspid, unicuspid or non-calcified aortic valve, severe aortic regurgitation (AR) and a native annulus size that is too small or too big relative to available TAVI devices (currently unsuitable annulus sizes include <18 mm or >29 mm for a MCV prosthesisand <18 mm or >27 mm for an ESV prosthesis) are some excluding factors.7–9 In addition, expected patient survival of less than one year, a dilated ascending aorta at the sinotubular junction (>45 mm for the MCV prosthesis) and the presence of apical thrombus are also considered to be contraindications.6
Differences in Transcatheter Heart Valve Design
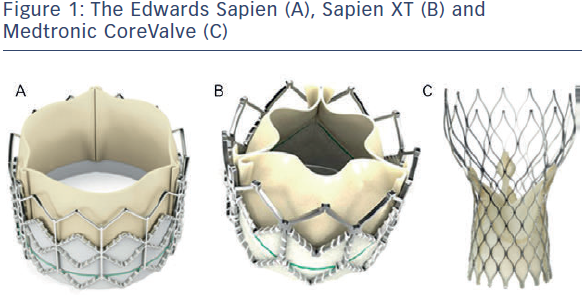
Edwards Sapien and Sapien XT Valve
The Edwards Sapien valve (ESV) (see Figure 1A) has been in use since 2006. It consists of a balloon-expandable stainless steel frame and three bovine pericardium cusps (leaflets) mounted onto a polyethylene terephthalate skirt (PET). Transfemoral delivery is facilitated via the RetroFlex 3 system, and the Ascendra system is used for transapical implantation. The newer generation Sapien XT valve, introduced in 2010 (see Figure 1B) consists of a balloon-expandable cobalt chromium frame with bovine pericardium cusps (leaflets). For trans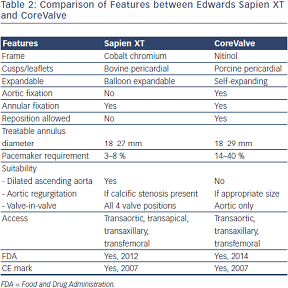 femoral implantation the valve is crimped onto the NovaFlex delivery catheter and introduced via a sheath into the femoral artery. Transapical and transaortic delivery are also possible using the Ascendra 2 or Ascendra plus systems. Rapid pacing is required during deployment as the valve is balloon expandable. Both valves are available in 23 mm and 26 mm sizes, although more recently, the Sapien XT has been introduced in a 29 mm size, enabling treatment of annular diameters between 18 and 27 mm. The newer generation Sapien 3 valve underwent first-in-human implantation in 2012. In comparison with the Sapien and Sapien XT valve this has a lower crimp profile and an additional outer PET skirt to reduce paravalvular regurgitation. This valve is not discussed further in this article.
femoral implantation the valve is crimped onto the NovaFlex delivery catheter and introduced via a sheath into the femoral artery. Transapical and transaortic delivery are also possible using the Ascendra 2 or Ascendra plus systems. Rapid pacing is required during deployment as the valve is balloon expandable. Both valves are available in 23 mm and 26 mm sizes, although more recently, the Sapien XT has been introduced in a 29 mm size, enabling treatment of annular diameters between 18 and 27 mm. The newer generation Sapien 3 valve underwent first-in-human implantation in 2012. In comparison with the Sapien and Sapien XT valve this has a lower crimp profile and an additional outer PET skirt to reduce paravalvular regurgitation. This valve is not discussed further in this article.
Medtronic CoreValve
The Medtronic CoreValve (MCV) consists of a nitinol self-expandable frame and porcine pericardial cusps (leaflets) mounted onto a porcine pericardium skirt (see Figure 1C). Nitinol, an alloy of nickel and titanium, exhibits martensitic transformation, which allows it to undergo a fully reversible solid-state transformation. Accordingly, at higher temperatures (e.g. body temperature) nickel exists as austenite, a strong cubic structure, whereas at lower temperatures (e.g. in an ice bath) it exists as martensite, a monoclinic crystal structure, exhibiting superelasticity, making it 10–30 times more elastic than other metals. This property allows it to be tightly compressed into a delivery sheath for implantation via the transarterial route (aortic or femoral).10 Currently, sizes 26, 29 and 31 mm are available. The next generation CoreValve Evolut valve is now available in 23 mm, and once in routine use, the range of treatable annular diameters will be between 17 and 29 mm. Table 2 details the differences between the ESV and MCV.
Alia Noorani - Consultant Edwards Lifesciences, Department of Cardiothoracic Surgery, Papworth Hospital, Cambridge
Vinayak Bapat - Consultant Cardiac Surgeon, Department of Cardiothoracic Surgery, St Thomas’ Hospital, Guy’s & St Thomas’ NHS Foundation Trust, London, UK