Indications for Each Valve Type
There are several factors to consider when contemplating a valve type and method of delivery. These include:
Native Annulus Size
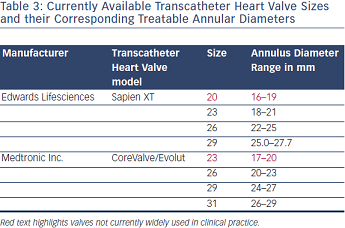 Accurate assessment of the native annulus should be at the forefront when considering a TAVI procedure. It is a given that an annulus size of <18 mm or >29 mm precludes any form of TAVI at present. Since there is no current imaging gold standards for annular assessment, a combination of several methods may be employed, including transthoracic and transoesophageal echocardiography in addition to computed tomography (CT) and magnetic resonance imaging (MRI).3 Available valve sizes and their appropriate corresponding native annulus sizes are given in Table 3. It is worth mentioning that at present, until smaller sizes of MCV (Evolut 23 mm) and ESV Sapien XT (20 mm) become available for routine clinical use, a size 23 mm ESV is suitable for a native annulus size of 18–20 mm and MCV 31 for 27–29 mm annular diameters.9 Finally a degree of relative oversize (5–10 %) is required with the ESV system to minimise the risk of paravalvular regurgitation.1
Accurate assessment of the native annulus should be at the forefront when considering a TAVI procedure. It is a given that an annulus size of <18 mm or >29 mm precludes any form of TAVI at present. Since there is no current imaging gold standards for annular assessment, a combination of several methods may be employed, including transthoracic and transoesophageal echocardiography in addition to computed tomography (CT) and magnetic resonance imaging (MRI).3 Available valve sizes and their appropriate corresponding native annulus sizes are given in Table 3. It is worth mentioning that at present, until smaller sizes of MCV (Evolut 23 mm) and ESV Sapien XT (20 mm) become available for routine clinical use, a size 23 mm ESV is suitable for a native annulus size of 18–20 mm and MCV 31 for 27–29 mm annular diameters.9 Finally a degree of relative oversize (5–10 %) is required with the ESV system to minimise the risk of paravalvular regurgitation.1
Cardiac Anatomy
In addition to annular diameter, a detailed assessment of cardiac anatomy is necessary as this can affect the choice of implant and method of delivery. Specifically, it is important to acknowledge specific structural variations:3
Peripheral Arterial Assessment
Accurate assessment of the peripheral arterial tree is crucial when considering an ideal method of delivery and the type of implant.3 Current methods of assessment include CT and peripheral angiography. In general, an ilio-femoral diameter <6 mm is considered unsuitable for transfemoral TAVI. The presence of ilio-femoral tortuosities, kinks in the aorta, existing stents, aneurysms or thrombi are also contraindications for transfemoral access, and alternative access via the transapical, transaortic or subclavian route have to be considered.
Risk of Annular Rupture
Aggressive balloon dilatation and oversizing during TAVI using the ESV has been associated with aortic root and annulus rupture.13 In a study of 37 consecutive patients with left ventricular outflow tract (LVOT) rupture during ESV deployment, a higher degree of sub-annular LVOT calcification (Agatston score), higher frequency of oversizing (by >20 %) as well as balloon post-dilatation were all associated with a higher risk of annular rupture.14 It is possible that the introduction of the Sapien 3 valve may reduce the risk of annular rupture as relatively less oversizing is required.
Differences in Outcomes
In addition to self-reported registry data, randomised data are available from the Placement of Aortic Transcatheter Valves (PARTNER) trial, which evaluated the ESV, and the CoreValve US Pivotal trial, which evaluated the MCV. The retrospective Pooled Rotterdam - Milano Collaboration (PRAGMATIC) study compared the two valves and the recent Comparison of Transcatheter Heart Valves in High Risk Patients With Severe Aortic Stenosis (CHOICE) study is the only randomised control trial of the two devices. In summary, although device specific complications are evident, there are no differences in clinical outcomes in terms of short- or long-term survival with use of either valve.15 Details of registry data and the relevant trials are presented below.
Edwards Sapien Valve
PARTNER TRIAL
The PARTNER trial consisted of two parallel arms. In cohort A, standard surgical aortic valve replacement (SAVR) was compared with TAVI in high-risk patients as determined by a STS score of >10 % and/or predicted operative mortality of 15 % or more. In cohort B, TAVI was compared with medical management (including balloon valvuloplasty) in inoperable patients with severe aortic stenosis. Patients enrolled into this cohort were deemed to have an operable risk of >50 %.
The one-year results from the trial demonstrated that in cohort A, TAVI and SAVR were found to be equivalent in terms of overall mortality, with all-cause mortality from TAVI at 24.2 % and 26.8 % from SAVR. Although the rate of stroke following TAVI was higher at 5.1 % compared with 2.4 % following SAVR, this result did not reach statistical significance. Results from cohort B confirmed that TAVI was superior to medical management, with the mortality rate from TAVI at 30.7 % compared with 50.7 % from medical treatment. There was, however, a higher rate of major vascular complications and stroke in the TAVI group compared with the conservatively managed group.
Review of the two-year data showed that the rate of stroke was 11.2 % with TAVI and 6.5 % with SAVR (p=0.05), and mortality in cohort A was 33.9 % from TAVI compared with 35.0 % from SAVR. Additionally, although paravalvular regurgitation was noted to be moderate in 7.0 % and severe in 1.9 % at one-year, this had decreased to 6.9 % and 0.9 %, respectively at two years, (p<0.001 for both intervals). Nonetheless, even mild paravalvular regurgitation was associated with an increased mortality (p<0.001).
Important findings from cohort B showed that mortality from TAVI was 43.3 % in the TAVI group compared with 68.0 % in the medical group, (p<0.05).15–17
SOURCE Registry
The Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) Registry is the largest series of consecutively ESV implanted TAVI patients with one-year outcomes. A total of 2,344 consecutive patients were enrolled in two cohorts to reflect trends overtime. Cohort 1 consisted of 1,038 patients enrolled at 32 European centres between November 2007 and January 2009. Transfemoral TAVI was undertaken in 463 patients and transapical in 575 patients. The overall one-year survival was 76.1 %; 72.1 % for transapical and 81.1 % for transfemoral access. The transapical group represented a higher-risk population, given that the logistic EuroSCORE for this group was significantly higher than that of the transfemoral TAVI patients.
Cohort 2 of the Registry consisted of a further 1,306 consecutive patients at 37 European centres enrolled between February 2009 and December 2009. Final results from the SOURCE registry indicated excellent results, with two-year survival at 67.7 % overall, similar stroke risks between the two cohorts of 2.5 % in cohort 1 and 2.8 % in cohort 2, which notably were lower than the 30 day stroke risk from transapical TAVI in cohort A of the PARTNER trial at 3.8 %.18,19
Medtronic CoreValve
ADVANCE Study
The MCV ADVANCE Study is the largest multicentre prospective MCV study comprising of 1,015 patients treated at 44 centres in 12 countries. The stroke rate in this group of patients was lower than the PARTNER A cohort at 4.5 %, and the one-year all-cause mortality rate was better than PARTNER A; 17.9 % compared with 24.2 %. These results were an improvement on previous pre-dated registry data such as FRANCE-2 and the UK registry (see below).20
CoreValve US Pivotal Trial
This prospective, randomised controlled multicentre trial of 795 patients in 45 centres in the US compared the MCV with SAVR in high-risk patients. The primary endpoint was the risk of death from any cause at one-year.
All-cause mortality at one-year was 14.2 % in the MCV group versus 19.1 % in the SAVR group. In addition, lower rates of cardiovascular and cerebrovascular events were noted in the TAVI group. In contrast to PARTNER A, this study did not show a higher rate of stroke post-TAVI when compared with rates post-SAVR.
The incidence of moderate to severe AR post-TAVI at one-year was 6.1 %, although this did not have an adverse effect on survival. In the majority of patients (76.2 %) discharged with moderate to severe AR, this had reduced to mild or none on one-year follow-up. Possible explanations given were the properties of nitinol, a higher placement and more accurate pre-TAVI assessment of the annulus with CT.21
Weighted Meta-analysis of Early and Late Clinical Outcomes after CoreValve Transcatheter Aortic Valve Implantation in Seven National Registries.This meta-analysis included 2,156 patients treated with the MCV prosthesis between 2007 and 2010 in seven countries. Reported results included early (30-day) outcomes including procedural success, stroke and pacemaker rates as well as one-year survival rates. Pooled procedural success was 97.8 %, the stroke rate was 2.8 % and the pacemaker implantation rate was 28.7 %. Early survival was 93.4 % and one-year mortality was 17.1 %. Limitations of this meta-analysis by virtue of its design included the variability in patient selection criteria, the non-standardised definitions of adverse events and the under reporting of clinical events.22
Alia Noorani - Consultant Edwards Lifesciences, Department of Cardiothoracic Surgery, Papworth Hospital, Cambridge
Vinayak Bapat - Consultant Cardiac Surgeon, Department of Cardiothoracic Surgery, St Thomas’ Hospital, Guy’s & St Thomas’ NHS Foundation Trust, London, UK