Implications for Exercise Recommendations for ARVD/C Patients and Family Members
Taken together these data convincingly argue that participation in vigorous or competitive endurance exercise leads to poor outcomes in ARVD/C patients. A recent international expert consensus statement on the treatment of ARVD/C57 integrated these data and recommended that ARVD/C patients be restricted from competitive and/or endurance sports (Class I recommendation). Restriction from other athletic activities with the possible exception of recreational low-intensity sports was also recommended (Class IIa). These recommendations are generally concordant with existing professional recommendations from Europe and North America.58–60
ARVD/C Pathogenesis – How do Exercise and Genotype Interact in Disease Pathogenesis?
The research described establishes the role of both genetic predisposition and exercise in ARVD/C pathogenesis and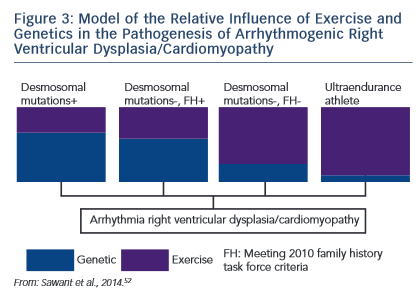 course. Our understanding of how these factors interact remains incomplete. An emerging paradigm suggests there is a threshold for phenotypic expression of ARVD/C with the relative amount of exercise necessary to reach the threshold varying based on genotype.52,61 As shown in the schematic (see Figure 3), we hypothesise that individuals born with very high genetic risk, such as carriers of multiple mutations, require little (or perhaps no) exercise to promote ARVD/C onset. At the other end of the spectrum, a series of studies by Heidbüchel et al. suggests ultra-endurance athletes exposed to massive amounts of exercise may develop a predominantly exercise-induced form of ARVD/C.62 This hypothesis, first proposed in 2003,63 was developed based on a clinical pattern of a high prevalence of RV arrhythmias and predominant RV dysfunction among high-level endurance athletes referred for evaluation of palpitations and other arrhythmia-associated symptoms. Further support for this concept came from their observation that among 41 athletes with definite or probable ARVD/C, only six had a definite or possible desmosomal mutation and few had a family history of disease.64 Furthermore, their mutation carriers had done significantly less exercise than the remaining cases suggesting less exercise was required for disease
course. Our understanding of how these factors interact remains incomplete. An emerging paradigm suggests there is a threshold for phenotypic expression of ARVD/C with the relative amount of exercise necessary to reach the threshold varying based on genotype.52,61 As shown in the schematic (see Figure 3), we hypothesise that individuals born with very high genetic risk, such as carriers of multiple mutations, require little (or perhaps no) exercise to promote ARVD/C onset. At the other end of the spectrum, a series of studies by Heidbüchel et al. suggests ultra-endurance athletes exposed to massive amounts of exercise may develop a predominantly exercise-induced form of ARVD/C.62 This hypothesis, first proposed in 2003,63 was developed based on a clinical pattern of a high prevalence of RV arrhythmias and predominant RV dysfunction among high-level endurance athletes referred for evaluation of palpitations and other arrhythmia-associated symptoms. Further support for this concept came from their observation that among 41 athletes with definite or probable ARVD/C, only six had a definite or possible desmosomal mutation and few had a family history of disease.64 Furthermore, their mutation carriers had done significantly less exercise than the remaining cases suggesting less exercise was required for disease 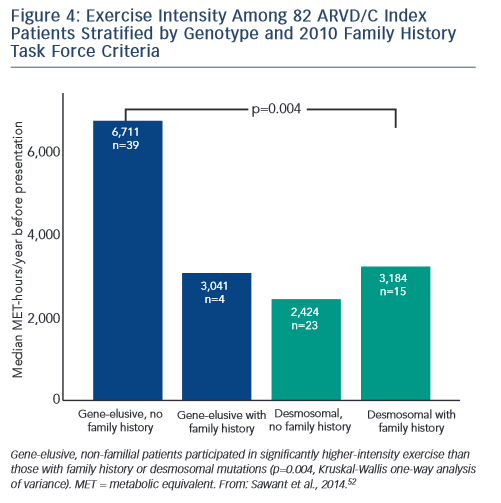 onset in the setting of a genetic predisposition.
onset in the setting of a genetic predisposition.
Sawant et al. recently confirmed and extended these findings via a study of 82 index cases, half of whom carried a desmosomal mutation.52 All the patients without desmosomal mutations were athletes (≥50 hours/year participation in a sport with high dynamic demand at vigorous intensity) in comparison to two-thirds of mutation carriers. Additionally, similar to the findings of Heidbüchel and colleagues, the patients without a desmosomal mutation had done considerably more intense exercise prior to clinical presentation. Sawant also found a relatively low prevalence of familial disease among cases without desmosomal mutations. Furthermore, as presented in Figure 4, gene-elusive cases with familial disease had performed exercise indistinguishable from that of desmosomal mutation carriers while the ARVD/C patients with neither a mutation nor a family history had done by far the most intense exercise.
While these studies suggest exercise plays a disproportionate role in the pathogenesis of ARVD/C cases without an apparent genetic predisposition, it is premature to conclude that this group of ARVD/C patients has an entirely acquired disease. In the study by Sawant et al., 10 % of cases with no identifiable mutation in the desmosomal genes, PLN or TMEM43 had clear evidence of familial disease by TFC. Second, ARVD/C is a rare disease (prevalence is estimated at 1/5,000)2 suggesting only a relatively small proportion of athletes are susceptible. One could speculate that this susceptibility stems from mutations in novel genes with lower penetrance or by combinations of rather low penetrant variants in both desmosomal and other genes. Other environmental factors as well as patient sex may also play a role.
Cynthia A James holds grants sponsored by the National Society of Genetic Counselors and the Barth Syndrome Foundation. The Johns Hopkins ARVD/C Program is supported by the Dr Francis P Chiaramonte Private Foundation, the Leyla Erkan Family Fund for ARVD Research, the Dr Satish Rupal and Robin Shah ARVD Fund at Johns Hopkins, the Bogle Foundation, the Healing Hearts Foundation, the Campanella family, the Patrick J Harrison Family, the Peter French Memorial Foundation and the Wilmerding Endowments.