Sleep-disordered Breathing
There are two main types of breathing abnormalities seen during SDB: obstructive sleep apnoea (OSA) and central sleep apnoea (CSA), which may manifest as Cheyne-Stokes respiration (CSR), particularly in patients with HF. OSA is the most common type of SDB in the general population, and occurs secondary to recurrent collapse of the 
The severity of SDB is reported as the number of respiratory events per hour of (estimated) sleep time (apnoea–hypopnoea index, AHI), with mild disease defined as an AHI of 5–15/h, moderate as 15–30/h and severe as ≥30/h. In addition, parameters documenting the extent of intermittent hypoxaemia (the oxygen desaturation index, ODI), sleep time or estimated sleep time spent with oxygen saturation <90 % and mean and minimal oxygen saturation are being used for this purpose.
SDB is characterised by intermittent hypoxia, reoxygenation, hypercapnia, arousals and sleep deprivation, as well as increased negative intrathoracic pressure swings when an obstruction of the upper airway is present. SDB is both a cause and a consequence of HF (see Figure 1).8 Potential mechanisms for increased cardiovascular risk in patients with OSA include sympathetic activation, changes in heart rate and blood pressure (BP) variability, vasoconstriction, oxidative stress, endothelial dysfunction, systemic inflammation, increased thrombotic risk, and functional problems including impaired diastolic function and increased wall stress, afterload and atrial size.9 In terms of the cardiovascular effects of CSA, it is thought that this form of sleep apnoea is most likely to be a consequence, rather than a cause, of HF.9,10 However, some of the physiologic effects of CSA are similar to those of OSA and therefore CSA also has the potential to initiate a cycle of events that lead to deterioration in cardiovascular function (see Figure 2).10,11 This includes increased sympathetic nervous system activity, greater cardiac electrical instability and low-frequency oscillations in blood pressure and heart rate.9,10 In contrast with OSA, CSA does not cause negative intrathoracic pressure swings, and the role of some of the other mechanisms contributing to increased cardiovascular risk in OSA, including inflammation, oxidative stress and endothelial dysfunction, remains to be determined.9,10
Sleep-disordered Breathing in Heart Failure
SDB is very common in patients with HF, much more common than in the general population, with prevalence rates of 50–75 %.12,13 SDB has been documented in patients with both HF-rEF14,15 or HF-pEF,16–18 with no difference in prevalence between groups19 and in patients with acute decompensated HF, where the prevalence can be even higher.20–22 One of the interesting features of SDB in patients with HF compared with general SDB patients is a relative lack of symptoms, especially of daytime somnolence,23–26 which coul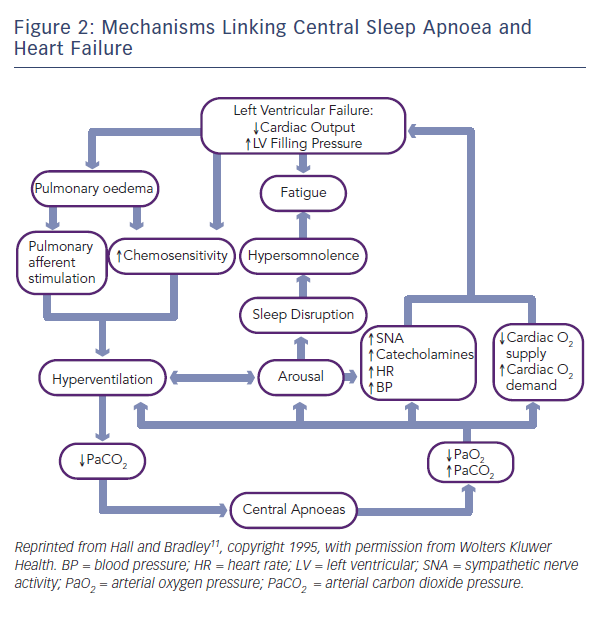
Approximately 20–45 % of patients with chronic HF have OSA.14,15 European HF guidelines recognise that sleep apnoea is of concern in patients with HF.3 OSA is independently associated with a worse prognosis in HF patients,24 even in those who are receiving maximal and optimal HF therapy.36 OSA is also highly prevalent in patients with HF-pEF, with a prevalence of 69–81 %.16,18 The predominant type of SDB in HF-pEF appears to be OSA, which occurs more often than CSA in these patients.16,18
While rarely found in the general population, CSA/CSR is a common SDB pattern seen in patients with chronic HF, with a prevalence of 25–40 %.15 The prevalence of CSA/CSR appears to increase as the severity of HF increases,12,16 and the severity of CSA/CSR seems to mirror cardiac dysfunction.37–39 Furthermore, CSA is independently associated with a worse prognosis in patients with HF, including increased mortality.24,26,36,40–42 Even mild CSA, with an AHI of ≥5/h has been associated with increased mortality.36 Although effective pharmacological9,35,38,43,44 and device-based45 treatment of HF may improve CSA/CSR, the negative impact of this form of SDB persists even in patients who are receiving maximal and optimal HF therapy, including cardiac resynchronisation.26,46 For patients who have persistent CSA despite optimal medical therapy, it may be necessary to consider other interventions.
At 44–97 %, the prevalence of CSA in patients with acute decompensated HF is even greater than that in those with stable HF.20–22 In addition, when present, CSA in acute decompensated HF patients is usually severe (AHI >30/h),21 and has been shown to be a predictor of readmission and mortality.47 It is interesting to note that optimal medical management, resolution of acute decompensation and return to baseline cardiopulmonary status are often not associated with a significant change in the severity of CSA.21,43,48,49 Insertion of a left ventricular assist device (LVAD) has been associated with improvements in SDB in refractory patients.50,51 These findings could indicate that severe CSA was present prior to the acute decompensation episode, and that more severe HF drives the higher prevalence and greater severity of CSA in these patients.48