Trastuzumab (Herceptin®)
Trastuzumab is a monoclonal antibody used in the treatment of HER2+ breast cancer. It exerts its actions by blocking the HER2 epidermal growth factor receptor, which is overexpressed in approximately 15–20 % of breast tumours and associated with more aggressive disease.65,66 Following phase III trials demonstrating significant improvements in both overall survival and risk of relapse, it was approved for clinical use in 1998.67
Introduction of trastuzumab into routine clinical practice, however, led to the unexpected finding of ‘off-target’ effects on the cardiovascular system. In one landmark trial, Slamon et al. demonstrated incidence rates of cardiac dysfunction of 27 % and New York Heart Association (NYHA) class III/IV symptoms of 16 % when trastuzumab was used in combination with both anthracyclines and cyclophosphamide.10
Pathophysiology of Trastuzumab-related Cardiomyopathy
The pathogenesis of trastuzumab cardiotoxicity is not completely understood, but appears to be related to blockage of HER2 receptor signals in cardiac myocytes – signalling that appears essential for cardiac myocyte repair.68
When given concurrently with anthracyclines, it has been proposed that the administration of anthracyclines results in initial oxidative damage to cardiac myocytes, resulting in those cells sustaining sufficient damage undergoing apoptosis or necrosis. The remaining injured myocytes undergo repair processes but remain temporarily vulnerable – the so-called ‘vulnerable window hypothesis’.69 In the presence of trastuzumab, inhibition of the normally upregulated HER2 results in loss of the usual repair mechanisms, driving the myocytes to further apoptosis and necrosis. In support of this theory are the findings of later studies examining the use of sequential anthracyclines and trastuzumab therapy. In these trials, initiation of a 3-week interval between treatments resulted in a reduction in the incidence of NYHA class III/IV HF to 3.8 %.12 With a longer 90-day interval, this reduced to just 0.6 %, with systolic dysfunction occurring in only 7 %70 compared with the 27 % reported by Slamon et al.10
In most instances, HF or LVSD related to trastuzumab is a sub-acute phenomenon, with the majority of cases being observed during treatment. It does, however, appear to be reversible after drug withdrawal and does not appear to be dose dependent.4
Risk Factors for Trastuzumab-related Cardiomyopathy
Apart from the well-recognised and documented risk in the setting of concurrent anthracycline therapy, risk factors fo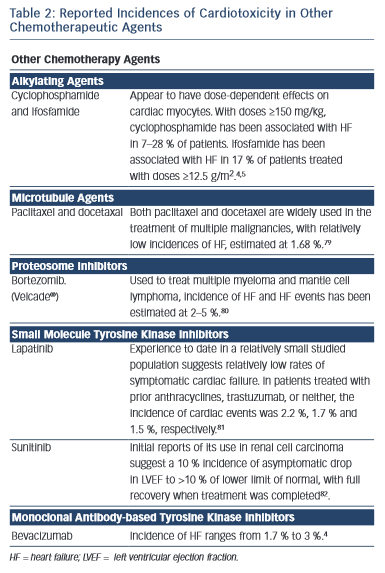 r the development of trastuzumab-induced HF or LVSD are less well defined. In part, this is due to the exclusion of both older patients and those with ‘traditional’ risk factors for cardiac disease from clinical trials. Several retrospective cohort analyses have attempted to address this matter.68,71,72 Results suggest those with borderline lower limit of normal LVEF, history of heart disease or prior treatment with anti-hypertensive medication and those of advanced age have the highest risk of cardiotoxicity.
r the development of trastuzumab-induced HF or LVSD are less well defined. In part, this is due to the exclusion of both older patients and those with ‘traditional’ risk factors for cardiac disease from clinical trials. Several retrospective cohort analyses have attempted to address this matter.68,71,72 Results suggest those with borderline lower limit of normal LVEF, history of heart disease or prior treatment with anti-hypertensive medication and those of advanced age have the highest risk of cardiotoxicity.
Prevention of Trastuzumab-related Cardiomyopathy
Few strategies have been adequately evaluated to determine their usefulness in preventing trastuzumab-induced HF or LVSD. Aside from delaying therapy until after treatment with anthracyclines is complete, possible strategies could include minimising trastuzumab treatment duration or the use of evidence-based HF medication.
Optimum duration for trastuzumab therapy remains unknown. Results from the Herceptin Adjuvant (HERA) trial comparing treatment over 12 and 24 months demonstrated no additional survival advantage with extended therapy, but did demonstrate continued risk of HF or LVSD.73 The Protocol of Herceptin Adjuvant with Reduced Exposure (PHARE) trial compared six versus 12 months of trastuzumab and failed to show non-inferiority of the shorter treatment administration but did demonstrate reduced incidence of cardiac events.74 Results from the Synergism or Long Duration (SOLD),75 Short-HER76 and PERSEPHONE77 trials are awaited to validate these findings.
No large randomised controlled trials have yet to report on the use of prognostically indicated HF medications to prevent HF or LVSD by trastuzumab. The Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research (MANTICORE) trial is due to address this issue by evaluating the efficacy of perindopril or bisoprolol for the prevention of LV remodelling in women with early breast cancer scheduled for chemotherapy and 1 year of trastuzumab.78
Other Chemotherapy Agents
Several other chemotherapy agents have also been associated with the development of heart failure (see Table 2).4,11,79–82
The research was supported by the National Institute for Health Research (NIHR) Clinical Research Facility at Guy’s & St Thomas’ NHS Foundation Trust and NIHR Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.